
(a)
Interpretation:
Product formed during the reaction of given anhydride with the given reagent has to be shown.
Concept Introduction:
Acid-catalyzed hydrolysis: In presence of strong acid such as
(b)
Interpretation:
Product formed during the reaction of given anhydride with the given reagent has to be shown.
Concept Introduction:
Ester Hydrolysis: Ester hydrolysis can be caused by acid and base.
Saponification: Ester hydrolysis taking place in presence of base such as
Acid-catalyzed hydrolysis: In presence of strong acid such as
(c)
Interpretation:
Product formed during the reaction of given anhydride with the given reagent has to be shown.
Concept Introduction:
Reduction:
LAH Reduction: The saturated/unsaturated aldehyde and ketones in the presence of sodium metal in LAH and carbonyl compound produced saturated alcohols. The keto group involves in the reduction process of LAH, this end up reducing to give the alcohols.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of
(d)
Interpretation:
Product formed during the reaction of given anhydride with the given reagent has to be shown.
Concept Introduction:
Ester Hydrolysis: Ester hydrolysis can be caused by acid and base.
Saponification: Ester hydrolysis taking place in presence of base such as
Acid-catalyzed hydrolysis: In presence of strong acid such as
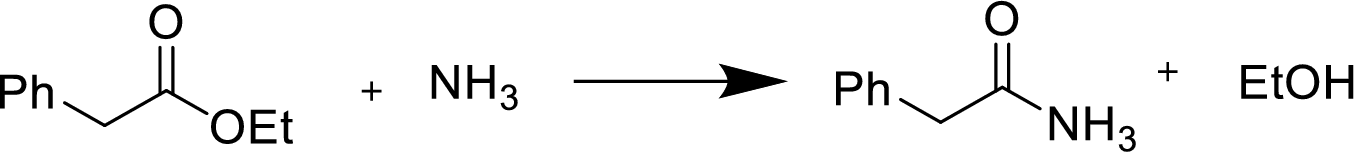
(e)
Interpretation:
Product formed during the reaction of given anhydride with the given reagent has to be shown.
Concept Introduction:
Reaction of an ester with ammonia or an
Treatment of an ester with ammonia or a primary or secondary amine gives an amide.
The nucleophilic addition of the ammonia or amine to the carbonyl carbon occurs followed by a proton transfer and a tetrahedral addition intermediate is formed. The intermediate can directly alkoxide and lose a proton to the alkoxide to give products.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Organic Chemistry
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

