
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.25P
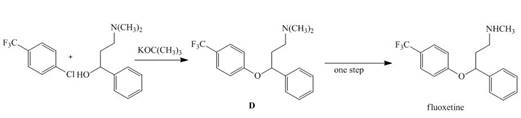
Draw a stepwise mechanism for the following reaction that forms ether D. D can be converted to the antidepressant fluoxetine (trade name Prozac) in a single step.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please add appropriate arrows, and tell me clearly where to add arrows, or draw it
What I Have Learned
Directions: Given the following reaction and the stress applied in each
reaction, answer the question below.
A. H2(g) + Cl2(g) 2 HCl(g)
Stress applied: Decreasing the pressure
1. What is the Keq expression?
2. What will be the effect in the number of moles of HCl(g)?
3. What will be the Equilibrium Shift or the reaction?
B.
Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g)
Stress applied: Increasing the temperature
1. What is the Keq expression?.
2. What will be the effect in the volume of water vapor collected?
3. What will be the Equilibrium Shift or the reaction?
C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat
Stress applied: Increasing the volume of the container
1. What is the Keq expression?.
2. What will be the effect in the amount of H₂O?
3. What will be the Equilibrium Shift or the reaction?
Consider the solubility products (Ksp values) for the following compounds:SrSO4 (Ksp = 7.6 x 10−7), BaSO4 (Ksp = 1.5 x 10−9), SrCO3 (Ksp = 7.0 x 10−10), BaCO3 (Ksp = 1.6 x 10−9)Which anion is the harder base, CO32− or SO42−? Justify your answer.
Chapter 18 Solutions
Organic Chemistry
Ch. 18 - Prob. 18.1PCh. 18 - Prob. 18.2PCh. 18 - Prob. 18.3PCh. 18 - Prob. 18.4PCh. 18 - Prob. 18.5PCh. 18 - Prob. 18.6PCh. 18 - Prob. 18.7PCh. 18 - Prob. 18.8PCh. 18 - Problem 18.9 Draw the product of each reaction
a....Ch. 18 - Prob. 18.10P
Ch. 18 - Prob. 18.11PCh. 18 - Prob. 18.12PCh. 18 - Prob. 18.13PCh. 18 - Problem 18.14 Draw all resonance structures for...Ch. 18 - Problem 18.15 Classify each substituent as...Ch. 18 - Prob. 18.16PCh. 18 - Problem 18.17 Label each compound as more or less...Ch. 18 - Problem 18.18 Rank the following compounds in...Ch. 18 - Prob. 18.19PCh. 18 - Problem 18.20 Draw the products of each...Ch. 18 - Prob. 18.21PCh. 18 - Problem 18.22 Draw the products formed when each...Ch. 18 - Problem 18.23 Devise a synthesis of each compound...Ch. 18 - Problem 18.24 Draw the products of each...Ch. 18 - Problem 18.25 Draw a stepwise mechanism for the...Ch. 18 - Problem 18.26 Draw the products of each...Ch. 18 - Prob. 18.27PCh. 18 - Prob. 18.28PCh. 18 - Problem 18.29 How could you use ethylbenzene to...Ch. 18 - Prob. 18.30PCh. 18 - Problem 18.31 What steps are needed to convert...Ch. 18 - Problem 18.32 Synthesize each compound from...Ch. 18 - Problem 18.33 Synthesize each compound from...Ch. 18 - Prob. 18.34PCh. 18 - 18.35 What is the major product formed by an...Ch. 18 - 18.36 Draw the products formed when phenol is...Ch. 18 - Problem 18.37 Draw the products formed when each...Ch. 18 - 18.38 Draw the products of each reaction.
a. d....Ch. 18 - 18.39 What products are formed when benzene is...Ch. 18 - 18.40 Draw the products of each reaction.
c.
d....Ch. 18 - 18.41 You have learned two ways to make an alkyl...Ch. 18 - 18.42 Draw the structure of A, an intermediate in...Ch. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - 18.45 Explain why each of the following reactions...Ch. 18 - Prob. 18.46PCh. 18 - 18.47 For each of the following substituted...Ch. 18 - 18.48 Consider the tetracyclic aromatic compound...Ch. 18 - 18.49 For each N-substituted benzene, predict...Ch. 18 - Prob. 18.50PCh. 18 - 18.51 Using resonance structures, explain why a...Ch. 18 - Prob. 18.52PCh. 18 - 18.53 Rank the aryl halides in each group in order...Ch. 18 - 18.54 Draw a stepwise mechanism for the following...Ch. 18 - Prob. 18.55PCh. 18 - 18.56 Draw a stepwise, detailed mechanism for the...Ch. 18 - Prob. 18.57PCh. 18 - 18.58 Draw a stepwise mechanism for the following...Ch. 18 - Prob. 18.59PCh. 18 - Prob. 18.60PCh. 18 - Prob. 18.61PCh. 18 - Prob. 18.62PCh. 18 - 18.63 Synthesize each compound from benzene and...Ch. 18 - Problem 18.64 Synthesize each compound from...Ch. 18 - Prob. 18.65PCh. 18 - Prob. 18.66PCh. 18 - Prob. 18.67PCh. 18 - Prob. 18.68PCh. 18 - Problem 18.69 Identify the structures of isomers A...Ch. 18 - Prob. 18.70PCh. 18 - Problem 18.71 Compound X (molecular formula ) was...Ch. 18 - 18.72 Reaction of p-cresol with two equivalents of...Ch. 18 - Prob. 18.73PCh. 18 - The NMR spectrum of phenol () shows three...Ch. 18 - Explain the reactivity and orientation effects...Ch. 18 - Prob. 18.76PCh. 18 - Prob. 18.77PCh. 18 - Prob. 18.78PCh. 18 - Prob. 18.79P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward(4 pts - 2 pts each part) A route that can be taken to prepare a hydrophobic (water-repellent) aerogel is to start with trichloromethylsilane, CH3SiCl3 as the silicon source. a. What is the chemical reaction that this undergoes to form a product with Si-OH groups? Write as complete of a chemical equation as you can. CI CI-SI-CH3 CI b. The formation of a byproduct is what drives this reaction - what is the byproduct (if you didn't already answer it in part (a)) and how/why does it form?arrow_forwardb) Circle the substrate that would not efficiently generate a Grignard reagent upon reaction with Mg in ether. CI Br ד c) Circle the Grignard reagents that contain incompatible functional groups. MgBr HO MgBr MgBr MgBr MgBr HO MgBrarrow_forward
- Q2: Predict all organic product(s), including stereoisomers when applicable. PCC OH a) CH2Cl2 Page 2 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) .OH Na2Cr2O7, H+ OH PCC CH2Cl2 c) OHarrow_forwardd) Circle the substrates that will give an achiral product after a Grignard reaction with CH3MgBr. Harrow_forwardQ4: Predict the organic products for the following reactions. Then draw curved arrow electron- pushing mechanism for the reactions. a) NaBH4 EtOH Page 4 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) 요 1. Et₂O H MgBr 2. H+, H₂Oarrow_forward
- Would Si(CH3)3F react with AgCl? If so, write out the balanced chemical equation. If not,explain why no reaction would take place.arrow_forwardNH3 reacts with boron halides (BX3 where X = F, Cl, Br, or I) to form H3N-BX3 complexes.Which of these complexes will have the strongest N-B bond? Justify your answerarrow_forward3Helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY