
a) PBr3
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with PBr3.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give
To give:
The products obtained when the alcohol shown is treated with PBr3.
Answer to Problem 22VC
The product obtained when the alcohol is treated with PBr3 is
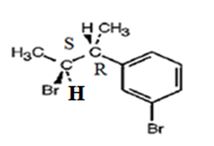
Explanation of Solution
The reaction of the alcohol with PBr3 is a bimolecular nucleophilic substitution (SN2) reaction. It takes place with inversion in configuration at the reaction centre to yield an alkyl bromide.
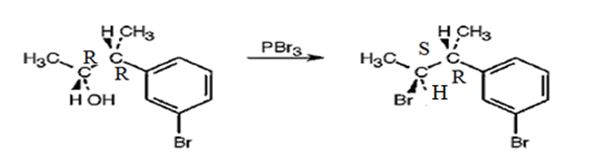
The product obtained when the alcohol is treated with PBr3 is

b) H2SO4
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with aqueous H2SO4.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated with aqueous H2SO4.
Answer to Problem 22VC
The products obtained when the alcohol is treated with aqueous H2SO4 are two isomeric alkenes (E) and (Z). E isomer is obtained as the major product.
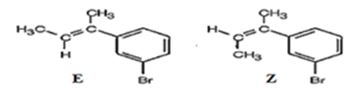
Explanation of Solution
The alcohol gets dehydrated when treated with aqueous H2SO4 yields the E and Z isomers of an alkene. The E isomer is more stable and obtained as the major product as the bulky groups are far away.
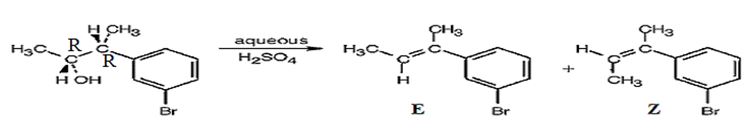
The products obtained when the alcohol is treated with aqueous H2SO4 isomeric alkenes (E) and (Z) are produced. E isomer is obtained as the major product.

c) SOCl2
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with SOCl2.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated with SOCl2.
Answer to Problem 22VC
The product obtained when the alcohol is treated with SOCl2 is
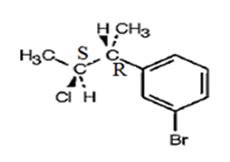
Explanation of Solution
The reaction of the alcohol with SOCl2 is a bimolecular nucleophilic substitution (SN2) reaction. It takes place with inversion in configuration at the reaction centre to yield an alkyl chloride.
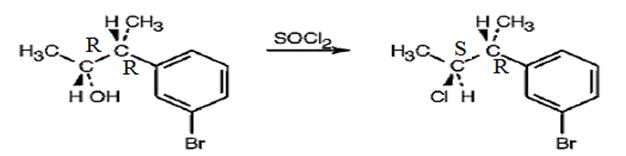
The product obtained when the alcohol is treated with SOCl2 is

d) Dess-Martin periodinate
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated with Dess-Martin periodinate.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated with Dess-Martin periodinate.
Answer to Problem 22VC
The product obtained when the alcohol is treated with Dess-Martin periodinate is
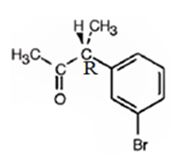
Explanation of Solution
The alcohol is a 20 in nature. Dess-Martin periodinate oxidizes it into a ketone.
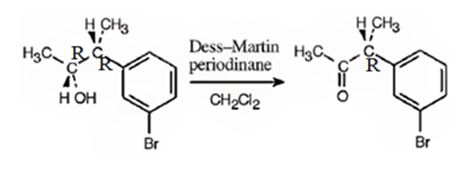
The product obtained when the alcohol is treated with Dess-Martin periodinate is

e) Br2, FeBr3
Interpretation:
The product obtained when 3-(m-bromophenyl)-2-butanol is treated Br2, FeBr3.
Concept introduction:
Alcohols when reacted with PBr3 yield the corresponding alkyl bromides along with H3BO3. When treated with aqueous H2SO4 they undergo dehydration to give alkenes. They react with SOCl2 to yield the corresponding alkyl halide along with SO2 and HCl. Dess-Martin periodinate oxidizes 20 alcohols to ketones. In aromatic electrophilic substitution reactions the alkyl group is an ortho and para directing and activating group. Bromine also is an ortho and para directing but deactivating group.
To give:
The products obtained when the alcohol shown is treated Br2, FeBr3.
Answer to Problem 22VC
The products obtained when the alcohol is treated with Br2, FeBr3 are
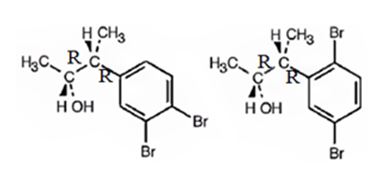
Explanation of Solution
The alcohol undergoes bromination in the benzene ring when treated with Br2, FeBr3. Both bromine and an alkyl group are attached to the ring. Both alkyl and Br are ortho and para directing, the alkyl is activating while the Br is deactivating. Hence during bromination the bromine enters into the ortho and para positions to yield the products.
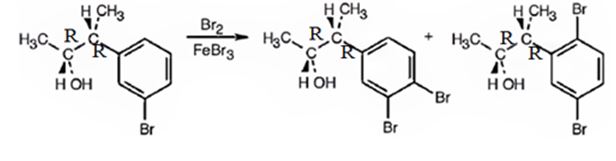
The products obtained when the alcohol is treated with Br2, FeBr3 are

Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry - With Access (Custom)
- Predict the major organic product(s) of the following reactions. Indicate which of the following mechanisms is in operation: SN1, SN2, E1, or E2.arrow_forward(c) (4pts) Mechanism: heat (E1) CH3OH + 1.5pts each _E1 _ (1pt) Br CH3OH (d) (4pts) Mechanism: SN1 (1pt) (e) (3pts) 1111 I H 10 Ill!! H LDA THF (solvent) Mechanism: E2 (1pt) NC (f) Bri!!!!! CH3 NaCN (3pts) acetone Mechanism: SN2 (1pt) (SN1) -OCH3 OCH3 1.5pts each 2pts for either product 1pt if incorrect stereochemistry H Br (g) “,、 (3pts) H CH3OH +21 Mechanism: SN2 (1pt) H CH3 2pts 1pt if incorrect stereochemistry H 2pts 1pt if incorrect stereochemistryarrow_forwardA mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixturearrow_forward
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

