
Student's Study Guide and Solutions Manual for Organic Chemistry
8th Edition
ISBN: 9780134066585
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17.1, Problem 5P
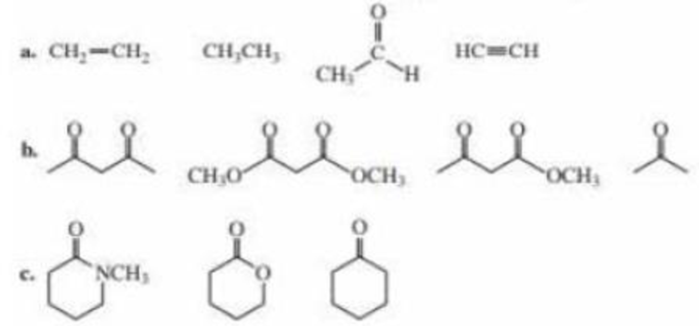
Rank the compounds in each of the following groups from strongest acid to weakest acid:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.
Draw mechanism
Does Avogadro's number have units?
Chapter 17 Solutions
Student's Study Guide and Solutions Manual for Organic Chemistry
Ch. 17.1 - Prob. 1PCh. 17.1 - Prob. 2PCh. 17.1 - Prob. 3PCh. 17.1 - Prob. 4PCh. 17.1 - Rank the compounds in each of the following groups...Ch. 17.2 - Explain why 92% of 2,4-pemtanedione exists as the...Ch. 17.3 - Draw the enol tautomers for each of the following...Ch. 17.3 - Prob. 8PCh. 17.4 - Prob. 9PCh. 17.4 - Prob. 10P
Ch. 17.5 - Show how the following compounds can be prepared...Ch. 17.6 - What compound is formed when a dilute solution of...Ch. 17.7 - Prob. 13PCh. 17.7 - Prob. 14PCh. 17.7 - How many stereoisomers are obtained from each of...Ch. 17.7 - Prob. 16PCh. 17.8 - Prob. 17PCh. 17.9 - Prob. 18PCh. 17.9 - What reagents should be used to prepare the...Ch. 17.10 - Prob. 20PCh. 17.10 - What aldehyde or ketone would be obtained when...Ch. 17.11 - Prob. 22PCh. 17.11 - How could you prepare the following compound using...Ch. 17.12 - Prob. 25PCh. 17.12 - What two carbonyl compound are required for the...Ch. 17.12 - Propose a mechanism for the following reaction:Ch. 17.13 - Draw the products of the following reactions:Ch. 17.13 - Prob. 29PCh. 17.13 - Prob. 30PCh. 17.14 - Prob. 31PCh. 17.15 - Write the mechanism for the reaction of a...Ch. 17.15 - Prob. 33PCh. 17.15 - Prob. 34PCh. 17.15 - Draw the product of the reaction of each of the...Ch. 17.16 - Draw the product obtained by heating each pair of...Ch. 17.16 - What two carbonyl compounds are needed to...Ch. 17.17 - Prob. 38PCh. 17.18 - Prob. 39PCh. 17.18 - Prob. 40PCh. 17.19 - Prob. 41PCh. 17.20 - Prob. 43PCh. 17.21 - Propose a mechanism for the formation of...Ch. 17.21 - Prob. 45PCh. 17.21 - a. If the biosynthesis of palmitic acid were...Ch. 17.21 - Prob. 47PCh. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Number the following compounds in order of...Ch. 17 - The 1H NMR chemical shifts of nitromethane,...Ch. 17 - Prob. 52PCh. 17 - Draw the products of the following reactions: a....Ch. 17 - A racemic mixture of 2-methyl-1-phenyl-1-butanone...Ch. 17 - Draw the products of the following reaction:Ch. 17 - Prob. 56PCh. 17 - Prob. 57PCh. 17 - In the presence of excess base and excess halogen,...Ch. 17 - Identify A-L. (Hint: A shows three singles in its...Ch. 17 - Using cyclopentanone as the reactant, show the...Ch. 17 - Show how 4-methyl-3-hexanol can be synthesized...Ch. 17 - Show how the following compound can be synthesized...Ch. 17 - Show how the following compounds can be prepared...Ch. 17 - Prob. 64PCh. 17 - Prob. 65PCh. 17 - Indicate how each of the following compounds can...Ch. 17 - Prob. 67PCh. 17 - The ketone whose 1H NMR spectrum is shown here was...Ch. 17 - Indicate how the following compounds can be...Ch. 17 - Compound A with molecular formula C6H10 has two...Ch. 17 - Prob. 71PCh. 17 - Draw the products of the following reactions:Ch. 17 - Prob. 73PCh. 17 - a. Show how the amino acid alanine can be...Ch. 17 - Show how the following compounds can be...Ch. 17 - Prob. 76PCh. 17 - Explain why the following bromoketone forms...Ch. 17 - Prob. 78PCh. 17 - A carboxylic arid is formed when an -haloketone...Ch. 17 - An , -unsaturated carbonyl compound can be...Ch. 17 - What carbonyl compounds are required to prepare a...Ch. 17 - Prob. 82PCh. 17 - A Cannizzaro reaction is the reaction of an...Ch. 17 - Propose a mechanism for each of the following...Ch. 17 - The following reaction is known as the benzoni...Ch. 17 - Prob. 86PCh. 17 - Prob. 87PCh. 17 - Prob. 88PCh. 17 - Prob. 89PCh. 17 - Prob. 90PCh. 17 - Propose a mechanism for the following reaction:Ch. 17 - What reagents are required to convert the reactant...Ch. 17 - Starting with bromocyclohexane, how can each of...Ch. 17 - Describe how the following compounds can be...Ch. 17 - Prob. 4PCh. 17 - Describe three ways to synthesize the following...Ch. 17 - Explain why 92% of 2.4-pentanedione exists as the...Ch. 17 - Describe how the following compound can be...Ch. 17 - Prob. 8PCh. 17 - Prob. 9PCh. 17 - Prob. 10PCh. 17 - Show how the following compounds can be...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY