
Physics, Books a la Carte Edition (5th Edition)
5th Edition
ISBN: 9780134020853
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 45PCE
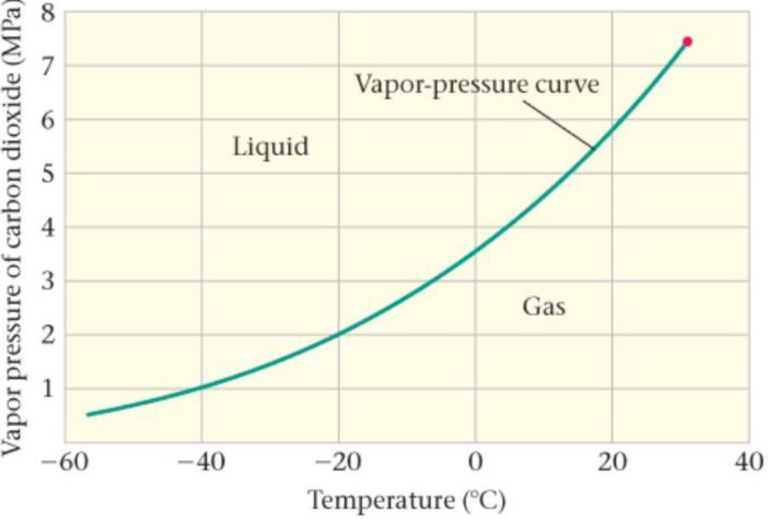
Predict/Calculate The Vapor Pressure of CO2 A portion of the vapor-pressure curve for carbon dioxide is given in Figure 17-36. (a) Estimate the pressure at which CO2 boils at 0 °C. (b) If the temperature is increased, does the boiling pressure increase, decrease, or stay the same? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
need help part e
Critical damping is the case where the mass never actually crosses over equilibrium position, but reaches equilibrium as fast as possible. Experiment with changing c to find the critical damping constant. Use the same initial conditions as in the last problem. Zoom in a bit to make sure you don't allow any oscillations to take place - even small ones.
Chapter 17 Solutions
Physics, Books a la Carte Edition (5th Edition)
Ch. 17.1 - Rank the following ideal-gas systems in order of...Ch. 17.2 - If the Kelvin temperature of a gas is doubled, by...Ch. 17.3 - A metal rod of a given initial length and...Ch. 17.4 - A portion of a substances phase diagram is shown...Ch. 17.5 - Which requires more heat: melting 100 kg of copper...Ch. 17.6 - An ice cube is placed in a cup of water. A few...Ch. 17 - How is the air pressure in a tightly sealed house...Ch. 17 - The average speed of air molecules in your room is...Ch. 17 - Is it possible to change both the pressure and the...Ch. 17 - Prob. 4CQ
Ch. 17 - A camping stove just barely boils water on a...Ch. 17 - An autoclave is a device used to sterilize medical...Ch. 17 - As the temperature of ice is increased, it changes...Ch. 17 - BIO Isopropyl alcohol is sometimes rubbed onto a...Ch. 17 - A drop of water on a kitchen counter evaporates in...Ch. 17 - (a) Is the number of molecules in one mole of N2...Ch. 17 - Predict/Explain If you put a helium-filled balloon...Ch. 17 - Two containers hold ideal gases at the same...Ch. 17 - Prob. 4PCECh. 17 - BIO After emptying her lungs, a person inhales 4.3...Ch. 17 - An automobile tire has a volume of 0.0185 m3. At a...Ch. 17 - Prob. 7PCECh. 17 - A compressed-air tank holds 0.500 m3 of air at a...Ch. 17 - Four ideal gases have the following pressures, P,...Ch. 17 - A balloon contains 3.9 liters of nitrogen gas at a...Ch. 17 - Prob. 11PCECh. 17 - Predict/Calculate A bicycle tire with a volume of...Ch. 17 - A 515-cm3 flask contains 0.460 g of a gas at a...Ch. 17 - Prob. 14PCECh. 17 - The air inside a hot-air balloon has an average...Ch. 17 - Prob. 16PCECh. 17 - Consider the system described in the previous...Ch. 17 - Prob. 18PCECh. 17 - Prob. 19PCECh. 17 - If the translational speed of molecules in an...Ch. 17 - At what temperature is the rms speed of H2 equal...Ch. 17 - Suppose a planet has an atmosphere of pure ammonia...Ch. 17 - Prob. 23PCECh. 17 - Prob. 24PCECh. 17 - Prob. 25PCECh. 17 - What is the temperature of a gas of CO2 molecules...Ch. 17 - The rms speed of a sample of gas is increased by...Ch. 17 - Prob. 28PCECh. 17 - A 380-mL spherical flask contains 0.065 mol of an...Ch. 17 - Prob. 30PCECh. 17 - A rock climber hangs freely from a nylon rope that...Ch. 17 - BIO To stretch a relaxed biceps muscle 2.5 cm...Ch. 17 - A 22-kg chimpanzee hangs from the end of a...Ch. 17 - The Marianas Trench The deepest place in all the...Ch. 17 - Four cylindrical rods with various cross-sectional...Ch. 17 - Predict/Calculate A steel wire 4.1 m long...Ch. 17 - BIO Spiderweb An orb weaver spider with a mass of...Ch. 17 - Predict/Calculate Two rods of equal length (0.55...Ch. 17 - A piano wire 0.82 m long and 0.93 mm in diameter...Ch. 17 - The formation of ice from water is accompanied by...Ch. 17 - Vapor Pressure for Water Figure 17-35 shows a...Ch. 17 - Using the vapor-pressure curve given in Figure...Ch. 17 - Prob. 43PCECh. 17 - Prob. 44PCECh. 17 - Predict/Calculate The Vapor Pressure of CO2 A...Ch. 17 - Phase Diagram for Water The phase diagram for...Ch. 17 - Phase Diagram for CO2 The phase diagram for CO2 is...Ch. 17 - Prob. 48PCECh. 17 - How much heat must be removed from 1.96 kg of...Ch. 17 - A heat transfer of 9.5 105 J is required to...Ch. 17 - How much heat must be added to 2.55 kg of copper...Ch. 17 - An ammonia refrigeration cycle involves the...Ch. 17 - Prob. 53PCECh. 17 - Prob. 54PCECh. 17 - Prob. 55PCECh. 17 - Figure 17-30 shows a temperature-versus-heat plot...Ch. 17 - Predict/Calculate Suppose the 1.000 kg of water in...Ch. 17 - Prob. 58PCECh. 17 - When you go out to your car one cold winter...Ch. 17 - A large punch bowl holds 3.99 kg of lemonade...Ch. 17 - A 155-g aluminum cylinder is removed from a liquid...Ch. 17 - An 825-g iron block is heated to 352 C and placed...Ch. 17 - Party Planning You are expecting to serve 32 cups...Ch. 17 - Predict/Calculate A 35-g ice cube at 0.0 C is...Ch. 17 - A 48-g block of copper at 12 C is added to 110 g...Ch. 17 - A 0 075-kg ice cube at 0.0 C is dropped into a...Ch. 17 - To help keep her barn warm on cold days, a farmer...Ch. 17 - CE As you go up in attitude, do you expect the...Ch. 17 - Prob. 69GPCh. 17 - Prob. 70GPCh. 17 - Prob. 71GPCh. 17 - Cooling Computers Researchers are developing heat...Ch. 17 - Prob. 73GPCh. 17 - Prob. 74GPCh. 17 - Evaporating Atmosphere Hydrogen gas evaporates...Ch. 17 - Prob. 76GPCh. 17 - A Boiling Geyser (a) The column of water that...Ch. 17 - A Melting Glacier (a) A glacier is made of ice of...Ch. 17 - Peter catches a 4 2-kg striped bass on a fishing...Ch. 17 - A steel ball (density=7860kg/m3) with a diameter...Ch. 17 - A lead brick with the dimensions shown in Figure...Ch. 17 - (a) Find the amount of heat that must be extracted...Ch. 17 - Mighty Ice Lift A tremendous force is generated...Ch. 17 - Orthopedic Implants Metals such as titanium and...Ch. 17 - Students on a spring break picnic bring a cooler...Ch. 17 - A 5.9-kg block of ice at 1.5 C slides on a...Ch. 17 - A cylindrical copper rod 37 cm long and 7.5 cm in...Ch. 17 - Prob. 88PPCh. 17 - Prob. 89PPCh. 17 - Prob. 90PPCh. 17 - Prob. 91PPCh. 17 - Referring to Example 17-17 (a) Find the final...Ch. 17 - Referring to Example 17-17 (a) Find the final...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define and discuss these terms: (a) synapsis, (b) bivalents, (c) chiasmata, (d) crossing over, (e) chromomeres,...
Concepts of Genetics (12th Edition)
Explain how you can determine whether fault N is older or younger than igneous intrusion J.
Applications and Investigations in Earth Science (9th Edition)
What global policy changes and what individual choices can help us sustain the planet that sustains us?
Biology: Life on Earth with Physiology (11th Edition)
Match the following cell types with their correct definition. _________Macrophage _________NK cell _________Eos...
Human Anatomy & Physiology (2nd Edition)
15. A good scientific hypothesis is based on existing evidence and leads to testable predictions. What hypothes...
Campbell Biology: Concepts & Connections (9th Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- NASA's KC-135 Reduced Gravity Research aircraft, affectionately known as the "Vomit Comet," is used in training astronauts and testing equipment for microgravity environments. During a typical mission, the aircraft makes approximately 30 to 40 parabolic arcs. During each arc, the aircraft and objects inside it are in free-fall, and passengers float freely in apparent "weightlessness." The figure below shows the altitude of the aircraft during a typical mission. It climbs from 24,000 ft to 30,850 ft, where it begins a parabolic arc with a velocity of 155 m/s at 45.0° nose-high and exits with velocity 155 m/s at 45.0° nose-low. 31 000 45° nose high 45° nose low 24 000 Zero g 65 Maneuver time (s) (a) What is the aircraft's speed (in m/s) at the top of the parabolic arc? 110.0 m/s (b) What is the aircraft's altitude (in ft) at the top of the parabolic arc? 2.04e+04 What is the initial height at the start of the parabolic arc? What is the initial velocity at this point? What is the final…arrow_forward12. What could we conclude if a system has a phase trajectory that sweeps out larger and larger area as time goes by?arrow_forwardneed help part darrow_forward
- A cab driver heads south with a steady speed of v₁ = 20.0 m/s for t₁ = 3.00 min, then makes a right turn and travels at v₂ = 25.0 m/s for t₂ = 2.80 min, and then drives northwest at v3 = 30.0 m/s for t3 = 1.00 min. For this 6.80-min trip, calculate the following. Assume +x is in the eastward direction. (a) total vector displacement (Enter the magnitude in m and the direction in degrees south of west.) magnitude direction For each straight-line movement, model the car as a particle under constant velocity, and draw a diagram of the displacements, labeling the distances and angles. Let the starting point be the origin of your coordinate system. Use the relationship speed = distance/time to find the distances traveled during each segment. Write the displacement vector, and calculate its magnitude and direction. Don't forget to convert min to s! m Model the car as a particle under constant velocity, and draw a diagram of the displacements, labeling the distances and angles. Let the…arrow_forwardî A proton is projected in the positive x direction into a region of uniform electric field E = (-5.50 x 105) i N/C at t = 0. The proton travels 7.20 cm as it comes to rest. (a) Determine the acceleration of the proton. magnitude 5.27e13 direction -X m/s² (b) Determine the initial speed of the proton. 8.71e-6 magnitude The electric field is constant, so the force is constant, which means the acceleration will be constant. m/s direction +X (c) Determine the time interval over which the proton comes to rest. 1.65e-7 Review you equations for constant accelerated motion. sarrow_forwardThree charged particles are at the corners of an equilateral triangle as shown in the figure below. (Let q = 2.00 μC, and L = 0.750 m.) y 7.00 με 60.0° L 9 -4.00 μC x (a) Calculate the electric field at the position of charge q due to the 7.00-μC and -4.00-μC charges. 112 Once you calculate the magnitude of the field contribution from each charge you need to add these as vectors. KN/CI + 64 × Think carefully about the direction of the field due to the 7.00-μC charge. KN/Cĵ (b) Use your answer to part (a) to determine the force on charge q. 240.0 If you know the electric field at a particular point, how do you find the force that acts on a charge at that point? mN Î + 194.0 × If you know the electric field at a particular point, how do you find the force that acts on a charge at that point? mNarrow_forward
- In the Donkey Kong Country video games you often get around by shooting yourself out of barrel cannons. Donkey Kong wants to launch out of one barrel and land in a different one that is a distance in x of 9.28 m away. To do so he launches himself at a velocity of 22.6 m/s at an angle of 30.0°. At what height does the 2nd barrel need to be for Donkey Kong to land in it? (measure from the height of barrel 1, aka y0=0)arrow_forwardFor which value of θ is the range of a projectile fired from ground level a maximum? 90° above the horizontal 45° above the horizontal 55° above the horizontal 30° above the horizontal 60° above the horizontalarrow_forwardA map from The Legend of Zelda: The Breath of the Wild shows that Zora's Domain is 7.55 km in a direction 25.0° north of east from Gerudo Town. The same map shows that the Korok Forest is 3.13 km in a direction 55.0° west of north from Zora's Domain. The figure below shows the location of these three places. Modeling Hyrule as flat, use this information to find the displacement from Gerudo Town to Korok Forest. What is the magnitude of the displacement? Find the angle of the displacement. Measure the angle in degrees north of east of Gerudo Town.arrow_forward
- Race car driver is cruising down the street at a constant speed of 28.9 m/s (~65 mph; he has a “lead” foot) when the traffic light in front of him turns red. a) If the driver’s reaction time is 160 ms, how far does he and his car travel down the road from the instant he sees the light change to the instant he begins to slow down? b) If the driver’s combined reaction and movement time is 750 ms, how far do he and his car travel down the road from the instant he sees the light change to the instant he slams on her brakes and car begins to slow down? c) If the driver’s average rate of acceleration is -9.5 m/s2 as he slows down, how long does it take him to come to a stop (use information about his speed of 28.9 m/s but do NOT use his reaction and movement time in this computation)? Please answer parts a-c. Show all work. For each question draw a diagram to show the vector/s. Show all the step and provide units in the answers. Provide answer to 2 decimal places unless stated otherwise.arrow_forwardBelow you will find 100 m split times for the American and France men’s 4x100 meter free style relay race during the 2008 Beijing Summer Olympics). Answer questions a-d. a) What was the total race time for each team, in seconds? b) Which team won the race? What was the difference in the teams’ times? c) What was the average speed for each team for the whole race? (provide answer to 3 decimal places). d) Calculate the average speed for each swimmer and report the results in a table like the one above. Remember to show the calculation steps. (provide answer to 3 decimal places). PLEASE SHOW ALL WORK AND STEPS.arrow_forwardNeed complete solution Pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY