
Conceptual Physics / MasteringPhysics (Book & Access Card)
12th Edition
ISBN: 9780321908605
Author: Paul G. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 31RCQ
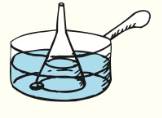
Place a Pyrex funnel mouth-down in a saucepan full of water so that the narrow tube of the funnel protrudes above water. Rest a part if the funnel on a nail or a coin so that water can get under it. Place the pan on a strove and watch the water as it begins to boil. Where do the bubbles form first? Why? As the bubbles rise, they expand rapidly and push water ahead of them. The funnel confines the water, which is forced up the tube and driven out at the top. Now do you know how a geyser and a coffee percolator work?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
SARET CRKS AUTOWAY
12. A stone is dropped from the top of a cliff. It is seen to hit the ground below
after 3.55 s. How high is the cliff?
13. A ball is dropped from rest at the top of a building that is 320 m tall. Assuming
no air resistance, what is the speed of the ball just before it strikes the ground?
14. Estimate (a) how long it took King Kong to fall straight down from the top
of the Empire State Building (280m high), and (b) his velocity just before
"landing".
Useful equations
For Constant Velocity:
V =>
D
X = V₁t + Xo
For Constant Acceleration:
Vr = V + at
X = Xo+Vot +
v=V+2a(X-Xo)
\prom = V +V
V velocity
t = time
D Distance
X = Final Position
Xo Initial Position
V = Final Velocity
Vo Initial Velocity
a = acceleration
For free fall
Yf
= Final Position
Yo Initial Position
g = 9.80
m
$2
For free fall:
V = V + gt
Y=Yo+Vo t +
+gt
V,² = V₁²+2g (Y-Yo)
V+Vo
Vprom=
2
6
Solve the problems
Chapter 17 Solutions
Conceptual Physics / MasteringPhysics (Book & Access Card)
Ch. 17 - Prob. 1RCQCh. 17 - Do the molecules in a liquid all have about the...Ch. 17 - What is evaporation?Ch. 17 - What is evaporation a cooling process?Ch. 17 - What is sublimation?Ch. 17 - Prob. 6RCQCh. 17 - Why is a steam burn more damaging than a burn from...Ch. 17 - Why do you feel uncomfortably warm on a hot and...Ch. 17 - Distinguish between humid and relative humidity.Ch. 17 - Why does water vapor in the air condense when the...
Ch. 17 - Why does warm, moist air from clouds when it...Ch. 17 - What is the basic difference between a cloud and...Ch. 17 - Distinguish between evaporation and boiling.Ch. 17 - Does increased atmospheric pressure increase or...Ch. 17 - Is it the boiling of water or the higher...Ch. 17 - Why doesn’t the water at the bottom of geyser boil...Ch. 17 - What happens to the water pressure at the bottom...Ch. 17 - Why doesn’t energy added to boiling water increase...Ch. 17 - When will water boil at a temperature lower...Ch. 17 - Prob. 20RCQCh. 17 - Why does increasing the temperature of a solid...Ch. 17 - Why does decreasing the temperature of a liquid...Ch. 17 - Why doesn’t water freeze at 00C when foreign ions...Ch. 17 - What happens to the hexagonal open structure of...Ch. 17 - Why doesn’t wire simply cut a block of ice in two...Ch. 17 - Does a liquid release energy or absorb energy when...Ch. 17 - Prob. 27RCQCh. 17 - Does the heat that is discharge at the...Ch. 17 - How many calories are needed to change the...Ch. 17 - Cite two reasons why firewalkers don’t burn their...Ch. 17 - Place a Pyrex funnel mouth-down in a saucepan full...Ch. 17 - Prob. 32RCQCh. 17 - Prob. 33RCQCh. 17 - Prob. 34RCQCh. 17 - Prob. 35RCQCh. 17 - Prob. 36RCQCh. 17 - The quantity of heat with temperature change is...Ch. 17 - Prob. 38RCQCh. 17 - Prob. 39RCQCh. 17 - Consider 50g of hot water at 800C poured into a...Ch. 17 - 50g chunk of 800C iron is dropped into a cavity in...Ch. 17 - Prob. 42RCQCh. 17 - Prob. 43RCQCh. 17 - 44. The heat of vaporization of ethyl alcohol is...Ch. 17 - Rank the boiling water temperatures from highest...Ch. 17 - From greatest to least, rank the energies needed...Ch. 17 - When you step out of a swimming pool on a hot, dry...Ch. 17 - Why is sweating an efficient mechanism for cooling...Ch. 17 - Why does blowing over hot soup cool the soup?Ch. 17 - What happens to the temperature of a pan of water...Ch. 17 - What is the source of energy that keeps the...Ch. 17 - An inventor claims to have developed a new perfume...Ch. 17 - Does a common electric fan cool the air in a room?...Ch. 17 - Prob. 54RCQCh. 17 - Prob. 55RCQCh. 17 - Prob. 56RCQCh. 17 - 57. Why are icebergs often surrounded by fog?
Ch. 17 - Prob. 58RCQCh. 17 - Prob. 59RCQCh. 17 - Prob. 60RCQCh. 17 - Prob. 61RCQCh. 17 - Prob. 62RCQCh. 17 - 63. A great amount of water vapor changes phase to...Ch. 17 - 64. Why does the temperature of boiling water...Ch. 17 - Prob. 65RCQCh. 17 - Prob. 66RCQCh. 17 - Prob. 67RCQCh. 17 - Prob. 68RCQCh. 17 - 69. Water will boil spontaneously in a vacuum—on...Ch. 17 - Prob. 70RCQCh. 17 - Prob. 71RCQCh. 17 - Prob. 72RCQCh. 17 - 73. If water that boils due to reduced pressure in...Ch. 17 - Prob. 74RCQCh. 17 - Prob. 75RCQCh. 17 - Prob. 76RCQCh. 17 - Prob. 77RCQCh. 17 - Prob. 78RCQCh. 17 - Prob. 79RCQCh. 17 - Prob. 80RCQCh. 17 - Prob. 81RCQCh. 17 - Prob. 82RCQCh. 17 - Prob. 83RCQCh. 17 - Prob. 84RCQCh. 17 - Prob. 85RCQCh. 17 - Prob. 86RCQCh. 17 - Prob. 87RCQCh. 17 - Prob. 88RCQCh. 17 - Prob. 89RCQCh. 17 - Prob. 90RCQCh. 17 - 91.Why is half-frozen fruit punch always sweeter...Ch. 17 - Prob. 92RCQCh. 17 - Prob. 93RCQCh. 17 - Prob. 94RCQCh. 17 - Prob. 95RCQCh. 17 - Prob. 96RCQCh. 17 - Prob. 97RCQCh. 17 - Prob. 98RCQCh. 17 - Prob. 99RCQCh. 17 - Prob. 100RCQCh. 17 - Prob. 101RCQCh. 17 - Prob. 102RCQCh. 17 - Prob. 103RCQCh. 17 - Prob. 104RCQCh. 17 - 105. When can you add heat to something without...Ch. 17 - Prob. 106RCQCh. 17 - 107. When can you withdraw heat from something...Ch. 17 - Discuss why water can issue from deep underwater...Ch. 17 - Prob. 109RCQCh. 17 - Prob. 110RCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 11 kg weight is attached to a spring with constant k = 99 N/m and subjected to an external force F(t) =-704 sin(5t). The weight is initially displaced 4 meters above equilibrium and given an upward velocity of 5 m/s. Find its displacement for t> 0. y(t) וןarrow_forward7. A race car accelerates from rest to 55 m s-1 in 5.0 seconds. The acceleration of the car Is m s-² 8. An object's speed increases uniformly from 10.5 km per hour to 99.8 km per hour in 2.41 seconds. Calculate the acceleration in m s-2 and express your answer to three significant figures. 9. The acceleration-time graph of a car is shown below. The initial speed of the car is 5.0 m s-1. # Acceleration (ms) 12 8.0- 4.0- 2.0 4.0 6.0 Time (s) Calculate the velocity of the car at t = 4.0 s. 3arrow_forwardNo chatgpt pls will upvotearrow_forward
- No chatgpt pls will upvotearrow_forwardProblem Seven. A football receiver running straight downfield at 5.60 m/s is 11.5 m in front of the quarterback when a pass is thrown downfield at an angle of 35.0° horizon. above the 8.) If the receiver never changes speed and the ball is caught at the same height from which it was thrown, find the distance between the quarterback and the receiver when the catch is made. (A) 21.3 (B) 17.8 (C) 18.8 (D) 19.9 (E) 67.5arrow_forwardPlease solve and answer the question correctly please. Thank you!!arrow_forward
- Please solve and answer the question correctly please. Thank you!!arrow_forwardPlease view both photos, and answer the question correctly please. Thank you!!arrow_forwardA thrown brick hits a window, but doesn't break it. Instead it reverses direction and ends down on the ground below the window. Since the brick didn't break the glass, we know: О The force of the brick on the glass > the force of the glass on the brick. О The force of the brick on the glass the force of the glass on the brick. = О The force of the brick on the glass < the force of the glass on the brick. О The brick didn't slow down as it broke the glass.arrow_forward
- Alexandra (wearing rubber boots for traction) is attempting to drag her 32.6-kg Golden Retriever across the smooth ice by applying a horizontal force. What force must she apply to move the dog with a constant speed of 0.950 m/s? ☐ 31.0 lb. ☐ 319 kg. ○ Zero. 32.6 kg.arrow_forwardThe figure shows a graph of the acceleration of an object as a function of the net force acting on it. The mass of this object, in grams, is closest to 11 a(m/s²) 8.0+ 6.0- 4.0- 2.0- 0+ F(N) 0.00 0.50 1.00 ☐ 130 ○ 8000 ☐ 89arrow_forwardValues that are within standard deviations represent measurements that are considered to be near the true value. Review the data from the lab and determine whether your data is within standard deviations. Report, using numerical values, whether your data for each angle is within standard deviations. An acceptable margin of error typically falls between 4% and 8% at the 95% confidence level. Review your data for each angle to determine whether the margin of error is within an acceptable range. Report with numerical values, whether your data for each angle is within an acceptable margin of error. Can you help explain what my data means in terms of the standard deviation and the ME? Thanks!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
A Level Physics – Ideal Gas Equation; Author: Atomi;https://www.youtube.com/watch?v=k0EFrmah7h0;License: Standard YouTube License, CC-BY