
Concept explainers
Stanozolol is an anabolic steroid that promotes muscle growth. Although stanozolol has been used by athletes and body builders, many physical and psychological problems result from prolonged use and it is banned in competitive sports.
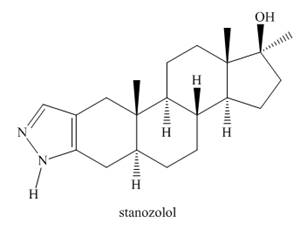
a. Explain why the nitrogen heterocycle—a pyrazole ring—is aromatic.
b. In what type of orbital is the lone pair on each
c. Draw all reasonable resonance structures for stanozolol.
d. Explain why the
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
PKG ORGANIC CHEMISTRY
- Can you please help me solve these homework questionsarrow_forwardAn einstein is the amount of energy needed to dissociate 1 mole of a substance. If we have 0.58 moles, do we need 0.58 einsteins to dissociate that substance?arrow_forwardIf the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.Data: Energy of each photon: 0.7835x10-18 J.arrow_forward
- If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



