
Concept explainers
(a)
Interpretation:
The complete mechanism that proceeds when the given species treated with triphenylphosphine followed by butyllithium is to be drawn.
Concept introduction:
The Wittig reagents can be generated from
Answer to Problem 17.53P
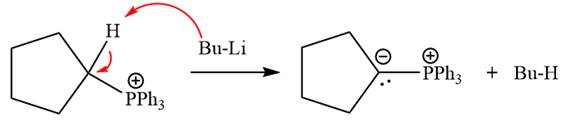
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
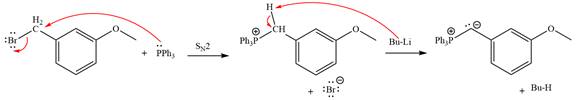
Explanation of Solution
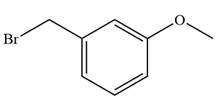
The given alkyl halide is
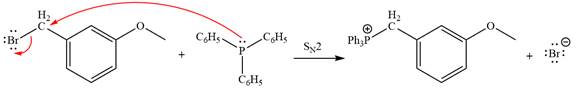
The Wittig reagent is generated from the given alkyl halide and triphenylphosphine followed by butyllithium. Triphenylphosphine,
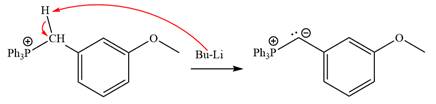
Lastly, the product obtained from the first step is treated with butyllithium to form Wittig reagent.
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
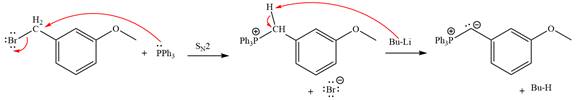
The complete mechanism for the given species treated with triphenylphosphine followed by butyllithium is drawn.
(b)
Interpretation:
The complete mechanism that proceeds when the given species treated with triphenylphosphine followed by butyllithium is to be drawn.
Concept introduction:
The Wittig reagents can be generated from alkyl halides. The alkyl halide is first reacted with triphenylphosphine and the product of that reaction is treated with a strong base. In the first step reaction fovors
Answer to Problem 17.53P
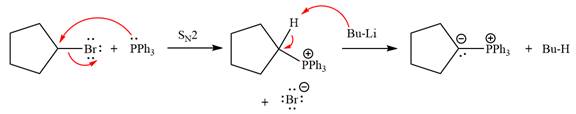
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
Explanation of Solution
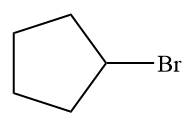
The given alkyl halide is
The Wittig reagent is generated from the given alkyl halide and triphenylphosphine followed by butyllithium. Triphenylphosphine,
Lastly, the product obtained from the first step is treated with butyllithium to form Wittig reagent.
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
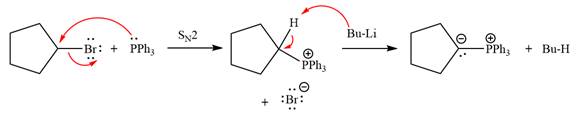
The complete mechanism for the given species treated with triphenylphosphine followed by butyllithium is drawn.
(c)
Interpretation:
The complete mechanism that proceeds when the given species treated with triphenylphosphine followed by butyllithium is to be drawn.
Concept introduction:
The Wittig reagents can be generated from alkyl halides. The alkyl halide is first reacted with triphenylphosphine and the product of that reaction is treated with a strong base. In the first step reaction fovors
Answer to Problem 17.53P
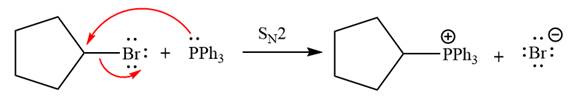
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
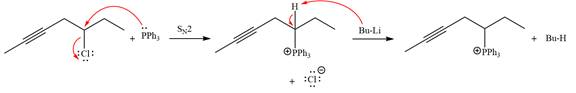
Explanation of Solution
The given alkyl halide is
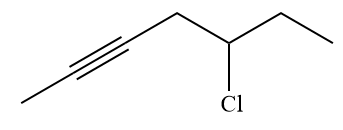
The Wittig reagent is generated from the given alkyl halide and triphenylphosphine followed by butyllithium. Triphenylphosphine,
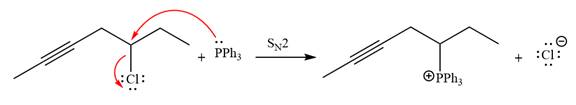
After that, the product obtained from the first step is treated with butyllithium to form Wittig reagent.
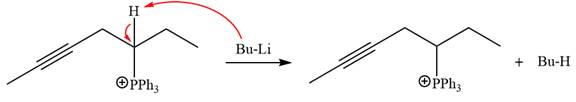
The complete mechanism of the given alkyl halide is treated with triphenylphosphine followed by butyllithium as shown below.
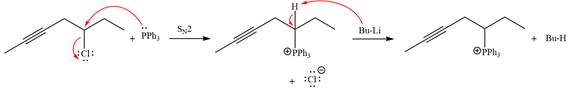
The complete mechanism for the given species treated with triphenylphosphine followed by butyllithium is drawn.
Want to see more full solutions like this?
Chapter 17 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Provide the unknown for the given data.arrow_forwardDraw the Lewis structures of two methanol (CH3OH) molecules and depict hydrogenbonding between them with dashed lines. Show all lone pairs. Provide a thorough analysis to apply concept idea into other problems.arrow_forwardSteps and explanation please.arrow_forward
- How could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forwardSteps and explanation please.arrow_forwardSteps and explanation on how to solve.arrow_forward
- Provide the unknown for the given data.arrow_forwardElectron Arrangement A. Fill in the following chart relating to levels, sublevels and orbitals. Levels (n) 1 Sublevels # of Orbitals per sublevel 2 3 4 # of Electrons per sublevel Total Electrons per level Complete: B. Answer the following questions related to levels, sublevels, orbitals and electrons. 1. How many sublevels are in energy level 2? 2. How many orbitals are in a 4f sublevel? 3. How many electrons can level 3 hold? 4. How many orbitals are in level 4? 5. How many electrons can sublevel 2p hold? 11arrow_forwardProvide the unknown for the given details.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
