
(a)
Interpretation:
The preparation method of benzyl methyl ether from toluene is to be stated.
Concept introduction:
Cyclic
Answer to Problem 17.28AP
The preparation method of benzyl methyl ether from toluene is shown below.
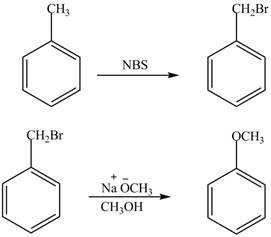
Explanation of Solution
For preparing benzyl methyl ether from toluene, toluene is first brominated.
For bromination of toluene,
The corresponding reaction sequences are shown below.
Figure 1
The preparation method of benzyl methyl ether from toluene is shown in Figure 1.
(b)
Interpretation:
The preparation method of
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of
Explanation of Solution
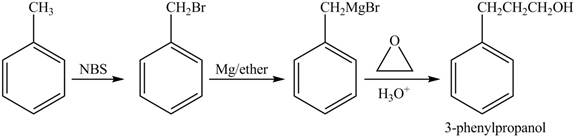
In given reaction, toluene is first brominated with NBS (N-bromosuccinamide). Brominated benzyl reacts with Grignard reagent and
The corresponding reaction sequences to obtain the desired product are shown below.

Figure 2
The preparation method of
(c)
Interpretation:
The preparation method of
Concept introduction:
The Lindlar’s catalyst is composed of
Answer to Problem 17.28AP
The preparation method of
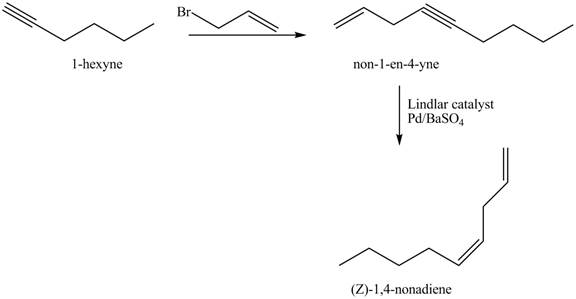
Explanation of Solution
The addition of
The corresponding reaction sequences to obtain the desired product are shown below.
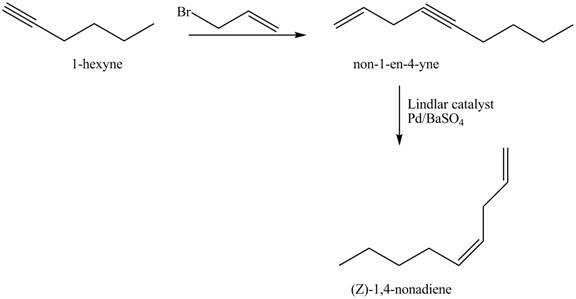
Figure 3
The preparation method of
(d)
Interpretation:
The preparation method of given compound from cyclopentene is to be stated.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
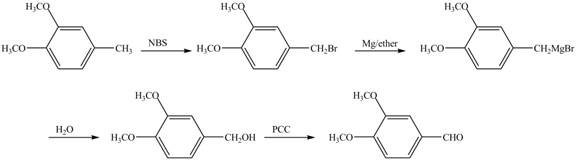
The preparation method of given compound from cyclopentene is shown below.
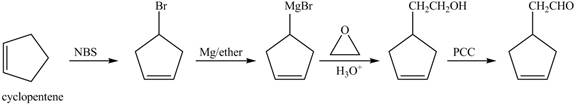
Explanation of Solution
Cyclopentene is first brominated and then on treatment with Grignard reagent and epoxide it gives corresponding alkyl alcohol. This alkyl alcohol is oxidized with mild reagent PCC to convert it into
The corresponding reaction sequences to obtain the desired product are shown below.
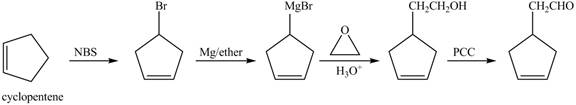
Figure 4
The preparation method of given compound from cyclopentene is shown in Figure 4.
(e)
Interpretation:
The preparation method of
Concept introduction:
The
Answer to Problem 17.28AP
The preparation method of
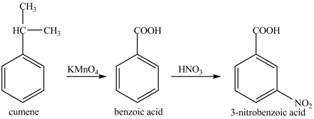
Explanation of Solution
Cumene has to be oxidized first and then its nitration is done to get the required product
Alkyl group is oxidized to gives benzoic acid. This benzoic acid is nitrated with
The corresponding reaction sequences to obtain the desired product are shown below.
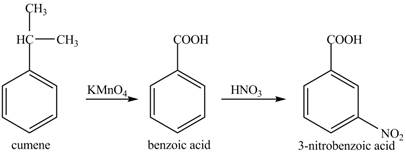
Figure 5
The preparation method of
(f)
Interpretation:
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is to be stated.
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as the electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 17.28AP
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown below.
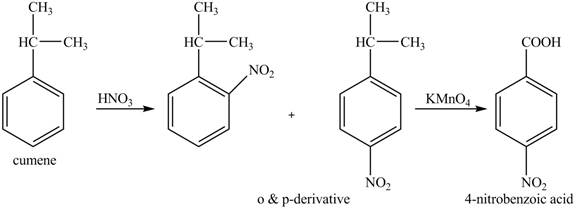
Explanation of Solution
As alkyl group is ortho and para directing, so, both the derivatives are formed. The reaction of cumene with nitric acid results in the formation of ortho and para nitro-cumene. The para nitro cumene is further oxidized to p-nitro benzoic acid with potassium permangnate.
The corresponding reaction sequences to obtain the desired product are shown below.
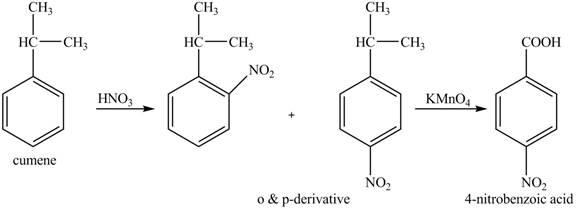
Figure 6
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown in Figure 6.
(g)
Interpretation:
The preparation method of
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of
Explanation of Solution
In the given reaction, at first, the substrate,
The corresponding reaction sequences to obtain the desired product are shown below.
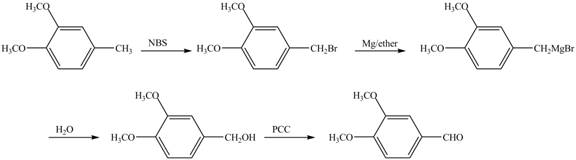
Figure 7
The preparation method of
(h)
Interpretation:
The preparation of given compound from cyclopentenol is to be given.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 17.28AP
The preparation of given compound from cyclopentenol is shown below.
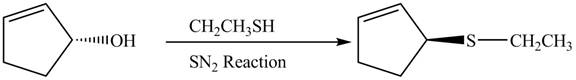
Explanation of Solution
The given reaction is a nucleophilic substitution reaction. The nucleophile attacks to replace the
This is a single step substitution nucleophile reaction as shown below.
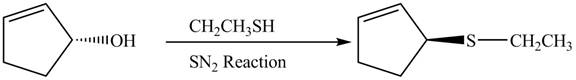
Figure 8
In this reaction, the stereochemistry of molecule changes as from one side the group is leaving and upon other side of the molecule, nucleophile attacks.
The preparation of given compound from cyclopentenol is shown in Figure 8.
Want to see more full solutions like this?
Chapter 17 Solutions
EBK ORGANIC CHEMISTRY
- What are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forward
- Potential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- Hi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forward
- Draw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





