
(a)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule is considered here.
The NMR signals for the
(a)
Explanation of Solution
Splitting in
In
In
.94ppm will the shift of
13.69ppm will the shift of
So the given values of the
Structural formula of the compound is;
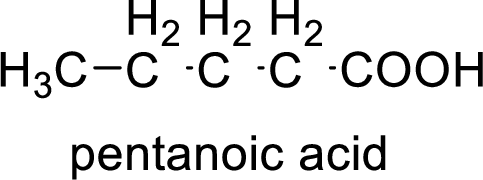
(b)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule is considered here.
The NMR signals for the
(b)
Explanation of Solution
Splitting in
In
In
1.08ppm will the shift of
30.69ppm will the shift of
So the given values of the
Structure formula of the compound is:
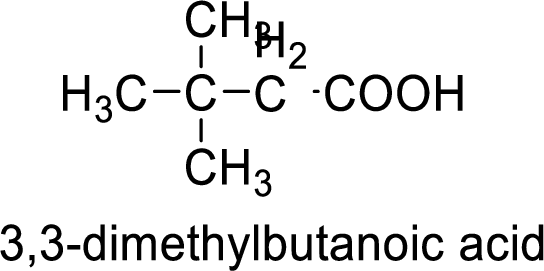
(c)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(c)
Explanation of Solution
Splitting in
In
In
.93ppm will the shift of
11.81ppm will be the shift of
So the given values of the
Structural formula of the product is;
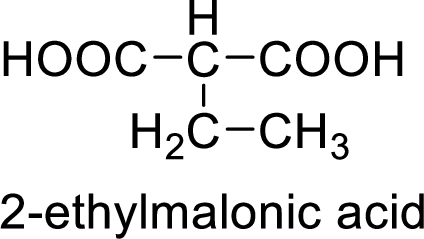
(d)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(d)
Explanation of Solution
Splitting in
In
In
1.29ppm will the shift of
22.56ppm will the shift of
So the given values of
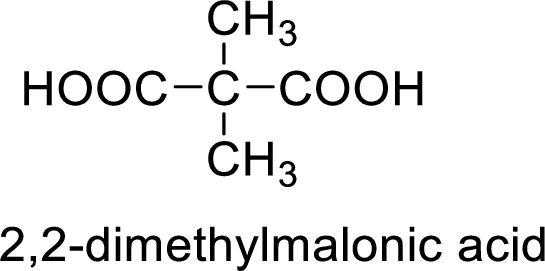
(e)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(e)
Explanation of Solution
Splitting in
In
In
1.91ppm will the shift of
18.11ppm will the shift of
So the given values of
Structural formula of the product;
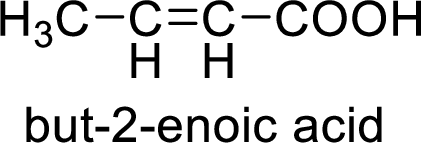
(f)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(f)
Explanation of Solution
Splitting in
In
In
2.34ppm will the shift of
34.02ppm will the shift of
So the given values of
Structure of the product
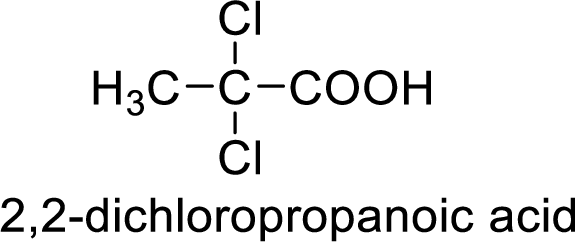
(g)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(g)
Explanation of Solution
Splitting in
In
In
1.42ppm will the shift of
13.69ppm will the shift of
So the given values of
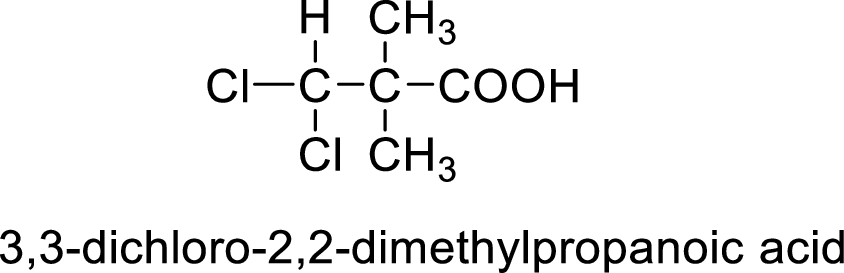
(h)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on an atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. Hydrogen nuclei with in the molecule are considered here.
The NMR signals for the
(h)
Explanation of Solution
Splitting in
In
In
97ppm will the shift of
13.24ppm will the shift of
So the given values of
Structural formula of the compound is;
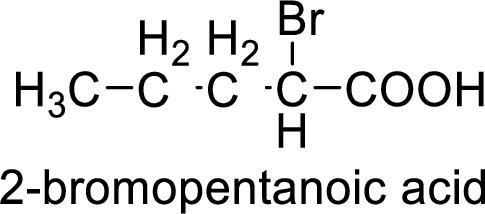
(i)
Interpretation:
Structural formula of the compound whose
Concept-Introduction:
Nuclear Magnetic Resonance Spectroscopy:
Nuclear Magnetic Resonance Spectroscopy or NMR Spectroscopy is a technique used to find the purity, content and molecular structure of the compound. Resonance transition between energy levels happens if electromagnetic radiation with specific frequency is applied on atomic nuclei placed in an electromagnetic field. This transition happens only if the frequency of applied radiation matches with the frequency of magnetic field, then it is to be in resonance condition. If an external magnetic field is applied spin gets excited from its ground state to the excited state by absorbing energy. The absorbed radio frequency is emitted back at the same frequency level, when the spin returns to its ground state. This radio frequency will give the NMR spectra. The plot of the spectra is between Intensity of the signal VS magnetic field. Trimethyl Silane ie, TMS is used as the reference. Chemical shift is a term which refers to the position in the spectrum. It is dependent on several factors like electron density around the proton, inductive effect etc.
It is also known as Proton Nuclear Magnetic Resonance. A hydrogen nucleus with in the molecule is considered here.
The NMR signals for the
(i)
Explanation of Solution
Splitting in
In
In
3.38ppm will the shift of
34.75ppm will the shift of
So the given values of
So the structure of the product is;
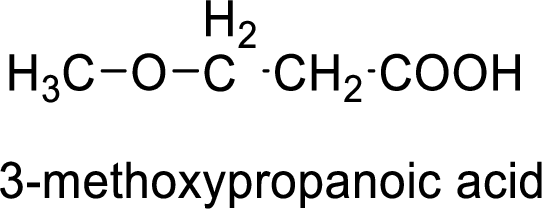
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- Please answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forward
- Consider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forward
- What is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

