
(a)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
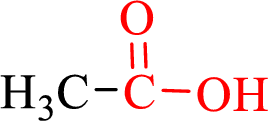
Nomenclature of
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the
alkane name with –oic acid.
Naming of compounds with two
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(a)
Explanation of Solution
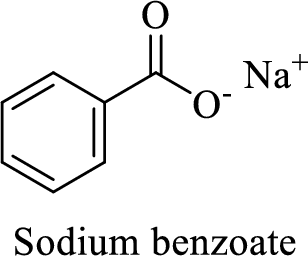
Name of the given salt is sodium benzoate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is a sodium salt of benzoic acid.
Thus,
The structural formula for this salt can be drawn as shown below,
(b)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
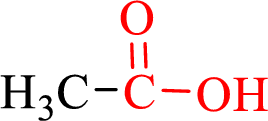
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(b)
Explanation of Solution
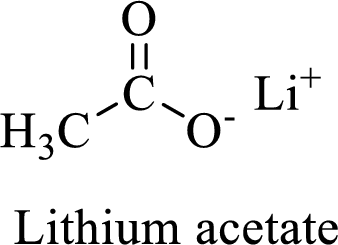
Name of the given salt is Lithium acetate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is a Lithium salt of acetic acid
Thus,
The structural formula for this compound can be drawn as shown below,
(c)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
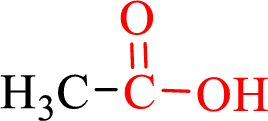
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(c)
Explanation of Solution
Name of the given salt is Ammonium acetate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is an ammonium salt of acetic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
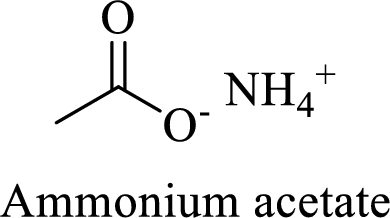
(d)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
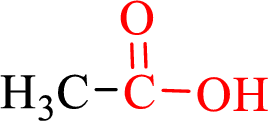
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(d)
Explanation of Solution
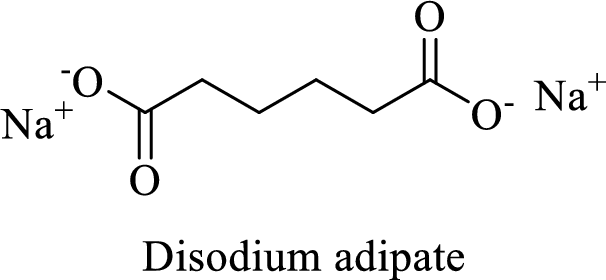
Name of the given salt is Disodium adipate.
From the name, we will get the following facts about the structure of the compound.
- ✓ The given compound is a sodium salt of adipic acid.
- ✓ Two sodium atoms are attached to the both carboxyl group of adipic acid.
The structure of adipic acid is shown below,
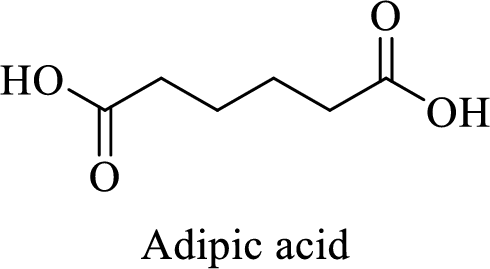
Thus,
The structural formula for this compound can be drawn as shown below,
(e)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
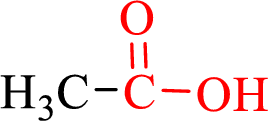
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(e)
Explanation of Solution
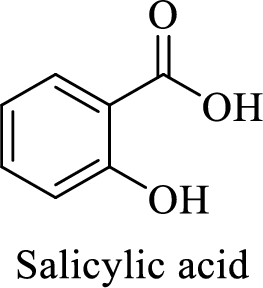
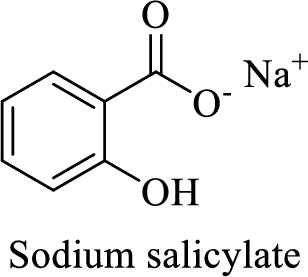
Name of the given salt is sodium salicylate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The compound is a sodium salt of salicylic acid.
Structure of salicylic acid is shown below,
Thus,
The structural formula for this compound can be drawn as shown below,
(f)
Interpretation: The structural formula for the given compound has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
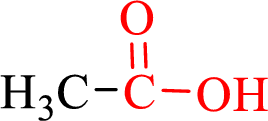
Nomenclature of carboxylic acid:
- • Find the Parent hydrocarbon chain.
- • Carboxyl carbon must be numbered first.
- • Replace the –e in the alkane name with –oic acid. If two or more carboxylic functional groups are present in the same compound then its number should be taken in to consideration and the prefix di, tri, tetra.. must be used.
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
Carboxylic salts are the water-soluble ammonium or alkali metal salts of carboxylic acids.
(f)
Explanation of Solution
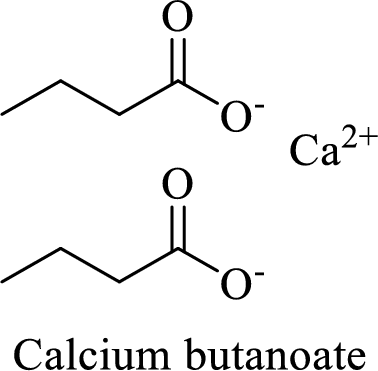
Name of the given salt is calcium butanoate.
From the name, we will get the following fact about the structure of the compound.
- ✓ The given compound is a calcium salt of butanoic acid.
Thus,
The structural formula for this compound can be drawn as shown below,
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- Explain how, based on physical gas adsorption isotherms, we can determine whether multi-walled C nanotubes are open at their ends. Explain this.arrow_forwardcan somone answer pleasearrow_forwardConstruct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forward
- Indicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forwardConsider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forward
- What constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forwardThe cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward
- The cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forwardName Section Score Date EXERCISE B pH, pOH, pка, AND PKD CALCULATIONS 1. Complete the following table. Solution [H+] [OH-] PH РОН Nature of Solution A 2 x 10-8 M B 1 x 10-7 M C D 12.3 6.8 2. The following table contains the names, formulas, ka or pka for some common acids. Fill in the blanks in the table. (17 Points) Acid Name Formula Dissociation reaction Ka pka Phosphoric acid H₂PO₁ H3PO4 H++ H₂PO 7.08 x 10-3 Dihydrogen H₂PO H₂PO H+ HPO 6.31 x 10-6 phosphate Hydrogen HPO₁ 12.4 phosphate Carbonic acid H2CO3 Hydrogen HCO 6.35 10.3 carbonate or bicarbonate Acetic acid CH,COOH 4.76 Lactic acid CH₂CHOH- COOH 1.38 x 10 Ammonium NH 5.63 x 10-10 Phenol CH₂OH 1 x 10-10 Protonated form CH3NH3* 3.16 x 10-11 of methylaminearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





