
a)
Interpretation:
The product of the reaction in which actophenone reacts with HNO3 and H2SO4 is to be predicted. The observed regiochemistry is to be explained by drawing the resonance structures of the intermediates.
Concept introduction:
The carbonyl group is an electron withdrawing group. Hence in
To predict:
The product of the reaction in which actophenone reacts with HNO3 and H2SO4.
To explain:
The observed regiochemistry by drawing the resonance structures of the intermediates.
Answer to Problem 77AP
Actophenone reacts with HNO3 and H2SO4 to produce m-nitroacetophenone.
Explanation of Solution
The electrophile, NO2+, attacks the aromatic ring in the first step to produce a resonance stabilized carbocation (sigma complex). In the second step the carbocation deprotonates to yield the product. For the meta attack the intermediate is more stabilized as shown.
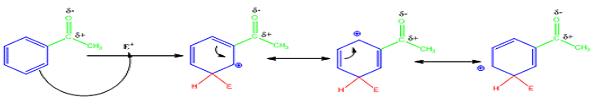
For ortho and para attack one of the resonance forms will be unstable as there will be a positive charge on the carbon to which the carbonyl group is attached.
Actophenone reacts with HNO3 and H2SO4 to produce m-nitroacetophenone.
b)
Interpretation:
The product of the reaction in which toluene reacts with isopropyl chloride in the presence of AlCl3 is to be predicted. The observed regiochemistry is to be explained by drawing the resonance structures of the intermediates.
Concept introduction:
Alkyl groups are an electron releasing groups. Hence during aromatic electrophilic substitution reactions the alkyl groups will activate the ring and orient the electrophile to the ortho and para positions.
To predict:
The product of the reaction in which toluene reacts with isopropyl chloride in the presence of AlCl3.
To explain:
The observed regiochemistry by drawing the resonance structures of the intermediates.
Answer to Problem 77AP
When toluene reacts with isopropyl chloride in the presence of AlCl3 the products obtained are o- and p-isopropylbenzenes. The p-isomer will predominate.
Explanation of Solution
The electrophile, (CH3)2CH+, attacks the aromatic ring in the first step to produce a resonance stabilized carbocation (sigma complex). In the second step the carbocation deprotonates to yield the product. For the ortho and para attacks, one of the the resonance structures has a positive charge on a carbon adjacent to the methyl group. This form is stabilized by electron release from the methyl group. Such stabilization is not possible for the meta attack. Hence a mixture of ortho-para products is produced in which the p-isomer predominates.
For ortho attack:
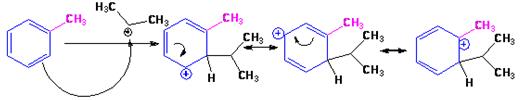
For para attack:
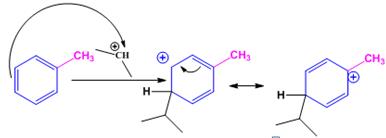
When toluene reacts with isopropyl chloride in the presence of AlCl3 the products obtained are o- and p-isopropylbenzenes. The p-isomer will predominate.
c)
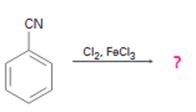
Interpretation:
The product of the reaction in which benzonitrile reacts with chlorine in the presence of FeCl3 is to be predicted. The observed regiochemistry is to be explained by drawing the resonance structures of the intermediates.
Concept introduction:
The nitrile group is an electron withdrawing group. Hence in aromatic electrophilic substitution reactions the nitrile group will deactivate the ring and orient the electrophile to the meta position.
To predict:
The product of the reaction in which benzonitrile reacts with chlorine in the presence of FeCl3.
To explain:
The observed regiochemistry by drawing the resonance structures of the intermediates.
Answer to Problem 77AP
When benzonitrile reacts with chlorine in the presence of FeCl3 meta chlorobenzonitrile will be produced.
Explanation of Solution
The electrophile, Cl+, attacks the aromatic ring in the first step to produce a resonance stabilized carbocation (sigma complex). In the second step the carbocation deprotonates to yield the product. For the meta attack the intermediate is more stabilized as shown.
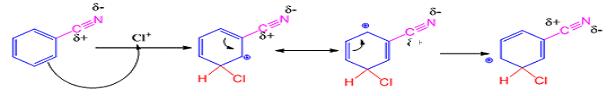
For ortho and para attack one of the resonance forms will be unstable as there will be a positive charge on the carbon to which the nitrile group is attached.
When benzonitrile reacts with chlorine in the presence of FeCl3 meta chlorobenzonitrile will be produced.
d)
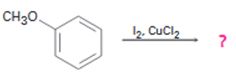
Interpretation:
The product of the reaction in which methoxy benzene reacts with iodine in the presence of CuCl2 is to be predicted. The observed regiochemistry is to be explained by drawing the resonance structures of the intermediates.
Concept introduction:
Methoxy group is an electron releasing group. Hence during aromatic electrophilic substitution reactions the methoxy group will activate the ring and orient the electrophile to the ortho and para positions.
To predict:
The product of the reaction in which benzene reacts with iodine in the presence of CuCl2.
To explain:
The observed regiochemistry by drawing the resonance structures of the intermediates.
Answer to Problem 77AP
When methoxybenzene reacts with iodine in the presence of CuCl2, the products obtained are o- and p-iodobenzenes.
Explanation of Solution
Iodine itself is unreactive toward aromatic rings. CuCl2 accelerate the iodination reaction by oxidizing iodine to a more powerful by electrophilic species that reacts like I+.
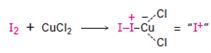
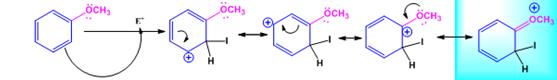
The electrophile, I+, attacks the aromatic ring in the first step to produce a resonance stabilized carbocation (sigma complex). In the second step the carbocation deprotonates to yield the product. For the ortho and para attacks, one of the the resonance structures has a positive charge on a carbon adjacent to the methoxy group. This form is stabilized by electron release from the methoxy group. Such stabilization is not possible for the meta attack. Hence a mixture of ortho-para products is produced.
Ortho attack:
Meta attack:
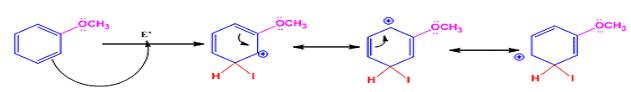
When methoxybenzene reacts with iodine in the presence of CuCl2, the products obtained are o- and p-iodobenzenes.
Want to see more full solutions like this?
Chapter 16 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Phenol is the starting material for the synthesis of 2,3,4,5,6-pentachlorophenol, known al-ternatively as pentachlorophenol, or more simply as penta. At one time, penta was widely used as a wood preservative for decks, siding, and outdoor wood furniture. Draw the structural formula for pentachlorophenol and describe its synthesis from phenol.arrow_forward12 Mass Spectrometry (d) This unknown contains oxygen, but it does not show any significant infrared absorption peaks above 3000 cm . 59 100- BO 40 Relative Abundance M(102) - 15 20 25 30 35 40 45 50 5 60 65 70 75 80 85 90 95 100 105 mizarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: H HO H HO H HO H H -OH CH2OH Click and drag to start drawing a structure. Х : Darrow_forward
- : Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH. Click and drag to start drawing a structure. P Darrow_forwardDraw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forward
- Take a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forwardAnswer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forward
- Nucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forwardSyntheses 22.35 Show how to convert toluene to these compounds. (a) -CH,Br (b) Br- -CH3 22.36 Show how to prepare each compound from 1-phenyl-1-propanone. 1-Phenyl-1-propanone ہتی. Br. (b) Br (racemic) 22.37 Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid. 22.38 Show reagents and conditions to bring about the following conversions. (a) 9 NH2 8 CO₂H NH2 CO₂Et (d) NO2 NH2 S NH₂ NO2 CHS CHarrow_forwardive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


