
Concept explainers
a)
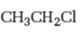
Interpretation:
Whether ethyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an
To state and explain:
Whether ethyl chloride is expected to undergo Friedal- Crafts reaction with or without rearrangement.
b)
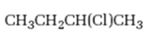
Interpretation:
Whether 2-chlorobutane is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift (particularly when a primary alkyl halide is used) can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether 2-chlorobutane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
c)
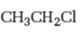
Interpretation:
Whether n-propyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether n-propyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement.
d)
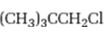
Interpretation:
Whether 1-chloro-2, 2-dimethylpropane is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether 1-chloro-2, 2-dimethylpropane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
e)
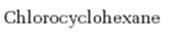
Interpretation:
Whether chlorocyclohexane is expected to undergo Friedal Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to yield an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether chlorocyclohexane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





