
Organic Chemistry - With Access (Custom)
9th Edition
ISBN: 9781337031745
Author: McMurry
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.SE, Problem 25VC
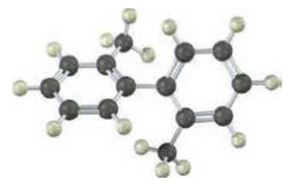
The following molecular model of a dimethyl-substituted biphenyl represents the lowest-energy conformation of the molecule. Why are the two benzene rings tilted at a 63� angle to each other rather than being in the same plane so that their p orbitals overlap? Why doesn't complete rotation around the single bond joining the two rings occur?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
9. compore the Following two Venctions IN
termy Of Ronction Rate and explan in
detail the reasoning that led to your conclusion
+He p₁₂ 11-
ㅐ 15
.. +He
H #H
H
/
H
b. Compare
the Following too reactions 14
terms of reaction Rate and explain in detail
the reasoning that led to your conclusion
Н
d-C-
tłu
Na
+2446
е
-ll +2n
"H
a.
•Write all of the possible products
For the Following ronction
А
-----
H
-
H
H
+ H₂0 H+
Н
b. in Rite the complete reaction Mechaniszn
For the Formation of each product.
·C. Suggest what Reaction conditions could
Result in each product being the major
Product of the veaction:
a. Write the product For each of the
Following reactions
H
6-836-6
레
+H₂ N
A
H
A-C-C=C-C-CH + 2 Na +2 NH3 -
H H
b. Write the reaction Mechanism For.
reaction
each
Chapter 16 Solutions
Organic Chemistry - With Access (Custom)
Ch. 16.1 - Prob. 1PCh. 16.2 - Propose a mechanism for the electrophilic...Ch. 16.2 - How many products might be formed on chlorination...Ch. 16.2 - When benzene is treated with D2SŪ4. deuterium...Ch. 16.3 - Prob. 5PCh. 16.3 - What is the major monosubstitution product from...Ch. 16.3 - Identify the carboxylic acid chloride that might...Ch. 16.4 - Rank the compounds in each of the following groups...Ch. 16.4 - Predict the major products of the following...Ch. 16.4 - Prob. 10P
Ch. 16.4 - Prob. 11PCh. 16.4 - Acetanilide is less reactive than aniline toward...Ch. 16.4 - Prob. 13PCh. 16.5 - At what position would you expect electrophilic...Ch. 16.5 - Show the major product(s) from reaction of the...Ch. 16.6 - The herbicide oxyfluorfen can be prepared by...Ch. 16.7 - Treatment of p-bromotoluene with NaOH at 300°C...Ch. 16.8 - Prob. 18PCh. 16.8 - Prob. 19PCh. 16.8 - Prob. 20PCh. 16.9 - Prob. 21PCh. 16.10 - Prob. 22PCh. 16.10 - Prob. 23PCh. 16.SE - Prob. 24VCCh. 16.SE - The following molecular model of a...Ch. 16.SE - Prob. 26VCCh. 16.SE - Prob. 27VCCh. 16.SE - Aromatic iodination can be carried out with a...Ch. 16.SE - Prob. 29MPCh. 16.SE - The carbocation electrophile in a Friede1-Crafts...Ch. 16.SE - Prob. 31MPCh. 16.SE - The nitroso group, —N=O, is one of the few...Ch. 16.SE - Triphenylmethane can be prepared by reaction of...Ch. 16.SE - Using resonance structures of the intermediates,...Ch. 16.SE - Benzene and alkyl -substituted benzenes can be...Ch. 16.SE - Prob. 36MPCh. 16.SE - Hexachlorophene, a substance used in the...Ch. 16.SE - Benzenediazonium carboxylate decomposes when...Ch. 16.SE - 4-Chloropyridine undergoes reaction with...Ch. 16.SE - Propose a mechanism to account for the following...Ch. 16.SE - In the Gatterman-Kochreaction, a formyl group...Ch. 16.SE - Treatment of p-tert-butylphenol with a strong acid...Ch. 16.SE - Benzyl bromide is converted into benzaldehyde by...Ch. 16.SE - Prob. 44MPCh. 16.SE - Prob. 45MPCh. 16.SE - Prob. 46APCh. 16.SE - Prob. 47APCh. 16.SE - Prob. 48APCh. 16.SE - Predict the major monoalkylation products you...Ch. 16.SE - Name and draw the major product(s) of...Ch. 16.SE - Prob. 51APCh. 16.SE - Prob. 52APCh. 16.SE - What product(s) would you expect to obtain from...Ch. 16.SE - Prob. 54APCh. 16.SE - How would you synthesize the following substances...Ch. 16.SE - Prob. 56APCh. 16.SE - Prob. 57APCh. 16.SE - Prob. 58APCh. 16.SE - Prob. 59APCh. 16.SE - Prob. 60APCh. 16.SE - Prob. 61APCh. 16.SE - Prob. 62APCh. 16.SE - Prob. 63APCh. 16.SE - How would you synthesize the following substances...Ch. 16.SE - Prob. 65APCh. 16.SE - Prob. 66APCh. 16.SE - Draw resonance structures of the intermediate...Ch. 16.SE - Prob. 68APCh. 16.SE - p-Bromotoluene reacts with potassium amide to give...Ch. 16.SE - Prob. 70APCh. 16.SE - Prob. 71APCh. 16.SE - Prob. 72APCh. 16.SE - Use your knowledge of directing effects, along...Ch. 16.SE - Identify the reagents represented by the letters...Ch. 16.SE - Phenols (ArOH) are relatively acidic, and the...Ch. 16.SE - Prob. 76APCh. 16.SE - Prob. 77APCh. 16.SE - Melamine, used as a fire retardant and a component...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License