
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.4, Problem 16.8P
Identify the N-terminal and C-terminal amino acid in each peptide.
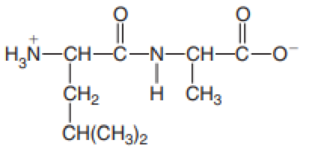
- a. Arg–His–Asn–Tyr
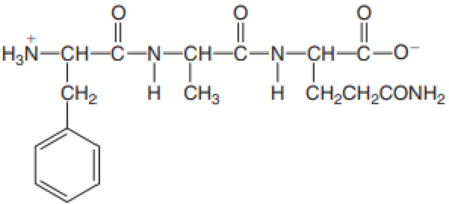
- b. Val–Thr–Pro–Phe
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the clean-pushing mechanism for the given ether synthesia from propanol in concentrated sulfurica140°C
by adding any mining aloms, bands, charges, nonbonding electron pairs, and curved arrows. Draw hydrogen bonded to
cayan, when applicable.
ore 11,0
HPC
Step 1: Draw curved arrows
Step 2: Complete the intend
carved
Q2Q
56
QQQ
Step 3: Complete the intermediate and add curved
Step 4: Modify the structures to draw the
QQQ
QQQ
6. In an experiment the following replicate set of volume
measurements (cm3) was recorded:
(25.35, 25.80, 25.28, 25.50, 25.45, 25.43)
A. Calculate the mean of the raw data.
B. Using the rejection quotient (Q-test) reject any
questionable results.
C. Recalculate the mean and compare it with the value
obtained in 2(a).
A student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there
are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from
the arrow.
• Is the student's transformation possible? If not, check the box under the drawing area.
• If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts,
inorganic reagents, or other important reaction conditions above and below the arrow.
• You do not need to balance the reaction, but be sure every important organic reactant or product is shown.
+
T
G
OH
де
OH
This transformation can't be done in one step.
Chapter 16 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 16.2 - In addition to the amino and carboxyl groups, what...Ch. 16.2 - How do the OH groups in Ser, Thr, and Tyr differ?Ch. 16.2 - Draw both enantiomers of each amino acid in...Ch. 16.2 - Which of the following amino acids is naturally...Ch. 16.3 - Draw the structure of the amino acid valine at...Ch. 16.3 - Identify the amino acid shown with all uncharged...Ch. 16.3 - Draw the positively charged, neutral, and...Ch. 16.4 - Identify the N-terminal and C-terminal amino acid...Ch. 16.4 - (a) Identify the N-terminal amino acid in the...Ch. 16.4 - Identify the individual amino acids in each...
Ch. 16.4 - Prob. 16.11PCh. 16.5 - Prob. 16.12PCh. 16.6 - Prob. 16.13PCh. 16.6 - Draw the structures of each pair of amino acids...Ch. 16.6 - The fibroin proteins found in silk fibers consist...Ch. 16.7 - Prob. 16.16PCh. 16.7 - Prob. 16.17PCh. 16.8 - Prob. 16.18PCh. 16.8 - Prob. 16.19PCh. 16.8 - Prob. 16.20PCh. 16.9 - Prob. 16.21PCh. 16.9 - Prob. 16.22PCh. 16.9 - The nerve gas sarin acts as a poison by covalently...Ch. 16.10 - Prob. 16.24PCh. 16 - Prob. 16.25UKCCh. 16 - Prob. 16.26UKCCh. 16 - For each amino acid: [1] draw the L enantiomer in...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - Label the regions of secondary structure in the...Ch. 16 - What type of interactions occur at each of the...Ch. 16 - Using the given representations for an enzyme and...Ch. 16 - Naturally occurring amino acids are L--amino...Ch. 16 - Why do neutral amino acids exist as zwitterions...Ch. 16 - The amino acid alanine is a solid at room...Ch. 16 - Why is phenylalanine water soluble but...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - Draw the structure of a naturally occurring amino...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - For each amino acid: [1] draw the l enantiomer in...Ch. 16 - Draw both enantiomers of each amino acid and label...Ch. 16 - Which of the following Fischer projections...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - For each amino acid: [1] give the name; [2] give...Ch. 16 - Draw the amino acid leucine at each pH: (a) 6; (b)...Ch. 16 - Draw the amino acid isoleucine at each pH: (a) 6;...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - Draw the structure of the neutral, positively...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For each tripeptide: [1] identify the N-terminal...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - For the given tripeptide: (a) identify the amino...Ch. 16 - Locate the peptide bond in the dipeptide shown in...Ch. 16 - Label the N-terminal and C-terminal amino acids in...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - Draw the structures of the amino acids formed when...Ch. 16 - What amino acids are formed by hydrolysis of the...Ch. 16 - Give the three-letter abbreviations for the amino...Ch. 16 - What is the difference between the primary and...Ch. 16 - What is the difference between the tertiary and...Ch. 16 - What type of intermolecular forces exist between...Ch. 16 - Which of the following pairs of amino acids can...Ch. 16 - List two amino acids that would probably be...Ch. 16 - List two amino acids that would probably be...Ch. 16 - Compare -keratin and hemoglobin with regards to...Ch. 16 - Compare collagen and myoglobin with regards to...Ch. 16 - When a protein is denatured, how is its primary,...Ch. 16 - Hydrogen bonding stabilizes both the secondary and...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Describe the function or biological activity of...Ch. 16 - Use the given representations for an enzyme,...Ch. 16 - Use the given representations for an enzyme and...Ch. 16 - How are enzyme inhibitors used to treat high blood...Ch. 16 - How are enzyme inhibitors used to treat HIV? Give...Ch. 16 - What structural feature in -keratin makes...Ch. 16 - Why does the -keratin in hair contain many...Ch. 16 - Why must vegetarian diets be carefully balanced?Ch. 16 - Why does cooking meat make it easier to digest?Ch. 16 - Sometimes an incision is cauterized (burned) to...Ch. 16 - Prob. 16.82APCh. 16 - How is sickle cell disease related to hemoglobin...Ch. 16 - The silk produced by a silkworm is a protein with...Ch. 16 - Explain the difference in the mechanism of action...Ch. 16 - How are blood enzyme levels used to diagnose...Ch. 16 - Explain why two amino acids aspartic acid and...Ch. 16 - Prob. 16.88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Macmillan Leaming Draw the major organic product of the reaction. 1. CH3CH2MgBr 2. H+ - G Select Draw Templates More H о QQarrow_forwardDraw the condensed structure of 3-hydroxy-2-butanone. Click anywhere to draw the first atom of your structure.arrow_forwardGive the expected major product of reaction of 2,2-dimethylcyclopropane with each of the following reagents. 2. Reaction with dilute H₂SO, in methanol. Select Draw Templates More CHC Erase QQQ c. Reaction with dilute aqueous HBr. Select Drew Templates More Era c QQQ b. Reaction with NaOCH, in methanol. Select Draw Templates More d. Reaction with concentrated HBr. Select Draw Templates More En a QQQ e. Reaction with CH, Mg1, then H*, H₂O 1. Reaction with CH,Li, then H', H₂Oarrow_forward
- Write the systematic name of each organic molecule: structure O OH OH name X ☐arrow_forwardMacmillan Learning One of the molecules shown can be made using the Williamson ether synthesis. Identify the ether and draw the starting materials. А со C Strategy: Review the reagents, mechanism and steps of the Williamson ether synthesis. Determine which of the molecules can be made using the steps. Then analyze the two possible disconnection strategies and deduce the starting materials. Identify the superior route. Step 6: Put it all together. Complete the two-step synthesis by selecting the reagents and starting materials. C 1. 2. Answer Bank NaH NaOH NaOCH, снен, сен, он Сиси, Сне (СН), СОН (Сн, Свarrow_forwardWrite the systematic name of each organic molecule: structure CH3 O CH3-CH-CH-C-CH3 OH HV. CH3-C-CH-CH2-CH3 OH CH3 O HO—CH, CH–CH—C CH3 OH 오-오 name X G ☐arrow_forward
- HI Organic Functional Groups Predicting the reactants or products of esterification What is the missing reactant in this organic reaction? HO OH H +回 + H₂O 60013 Naomi V Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click and drag to start drawing a structure. Explanation Check 1 2 #3 $ 4 2025 % ala5 'a :☐ G & 67 8 Ar K enter Accessible 9 Q W E R TY U 1 tab , S H J Karrow_forwardPlease help me with number 5 using my data and graph. I think I might have number 3 and 4 but if possible please check me. Thanks in advance!arrow_forwarddict the major products of this organic reaction. C Explanation Check 90 + 1.0₂ 3 2. (CH3)2S Click and drag f drawing a stru © 2025 McGraw Hill LLC. All Rights Reserved. • 22 4 5 7 8 Y W E R S F H Bilarrow_forward
- can someone draw out the reaction mechanism for this reaction showing all the curly arrows and 2. Draw the GPNA molecule and identify the phenylalanine portion. 3. Draw L-phenylalanine with the correct stereochemistryarrow_forwardWhat is the reaction mechanism for this?arrow_forwardPredict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. esc esc Explanation Check 2 : + + X H₁₂O + Х ง WW E R Y qab Ccaps lock shift $ P X Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil T FR F18 9 G t K L Z X V B N M control opption command command T C darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY