
Concept explainers
a)
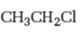
Interpretation:
Whether ethyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an
To state and explain:
Whether ethyl chloride is expected to undergo Friedal- Crafts reaction with or without rearrangement.
b)
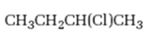
Interpretation:
Whether 2-chlorobutane is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift (particularly when a primary alkyl halide is used) can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether 2-chlorobutane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
c)
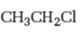
Interpretation:
Whether n-propyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether n-propyl chloride is expected to undergo Friedal-Crafts reaction with or without rearrangement.
d)
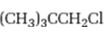
Interpretation:
Whether 1-chloro-2, 2-dimethylpropane is expected to undergo Friedal-Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to produce an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether 1-chloro-2, 2-dimethylpropane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
e)
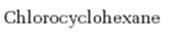
Interpretation:
Whether chlorocyclohexane is expected to undergo Friedal Crafts reaction with or without rearrangement is to be stated and explained.
Concept introduction:
Friedal-Crafts reaction is an electrophilic substitution reaction in which an aromatic compound reacts with an alkyl chloride in the presence of AlCl3 to yield an alkylbenzene. The electrophile in this reaction is an alkyl carbocation. The skeletal rearrangement of the alkyl carbocation electrophile either through a hydride shift or alkyl shift, particularly when a primary alkyl halide is used, can lead to the formation of rearranged product also in this reaction.
To state and explain:
Whether chlorocyclohexane is expected to undergo Friedal-Crafts reaction with or without rearrangement.
Trending nowThis is a popular solution!

Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





