
Acetic acid, CH3CO2H, can form a dimer, (CH3CO2H)2, in the gas phase..
The dimer is held together by to hydrogen bonds with a total strength of 66.5 kJ per mole of dimer.
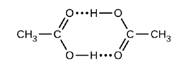
At 25 °C, the equilibrium constant for the dimerization is
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Chemistry (OER)
Additional Science Textbook Solutions
Applications and Investigations in Earth Science (9th Edition)
Chemistry: The Central Science (14th Edition)
Campbell Biology (11th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Microbiology with Diseases by Body System (5th Edition)
- Show work..don't give Ai generated solutionarrow_forwardShow work..don't give Ai generated solutionarrow_forwardPheromone G of the maize stalk borer, chilo partelus, can be synthesized based on the partial scheme shown below. Complete the scheme by identifying the structures of the intermediate compounds A, B, C, D, E, F and pheromone G. Indicate stereochemistry where relevantarrow_forward
- Q8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor. одarrow_forwardQ9: Explain why compound I is protonated on O while compound II is protonated on N. NH2 DD I II NH2arrow_forwardComplete the following reaction by identifying the principle organic product of the reactionarrow_forward
- Denote the dipole for the indicated bonds in the following molecules. ✓ H3C CH3 B F-CCl3 Br-Cl H3C —Si(CH3)3 CH3 OH HO HO H HO OH vitamin Carrow_forward(a) What is the hybridization of the carbon in the methyl cation (CH3*) and in the methyl anion (CH3)? (b) What is the approximate H-C-H bond angle in the methyl cation and in the methyl anion?arrow_forward10:16 ☑ Vo)) Vo) 4G LTE 76% Complete the following reaction by identifying the principle organic product of the reaction. HO OH ↑ CH2N2 OH ? ○ A. 01 N₂H2C OH ОН B. HO OCH3 OH ○ C. HO OH ŎCH₂N2 ○ D. H3CO OH он Quiz navigation 1 2 3 4 5 11 12 Next page 10 6 7 8 9 10arrow_forward
- Which one of the following statements explain why protecting groups are referred to as “a necessary evil in organic synthesis”? Question 12Select one or more: A. They increase the length and cost of the synthesis B. Every synthesis employs protecting groups C. Protecting group have no role to play in a synthesis D. They minimize the formation of side productsarrow_forwardWhich of the following attributes is a key advantage of the chiral auxiliary approach over the chiral pool approach in asymmetric synthesis? Question 10Select one: A. Chiral auxiliaries are cheaper than chiral pool substrates B. Chiral auxiliary can be recovered and recycled unlike chiral pool substrates. C. The use of chiral auxiliaries provide enantiopure products, while chiral pool reactions are only enantioselective D. The chiral auxiliaries are naturally occurring and do not require synthesisarrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. CH3 CH3 H3C HO: CI:arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





