
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
6th Edition
ISBN: 9781260325294
Author: SMITH
Publisher: RENT MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 52P
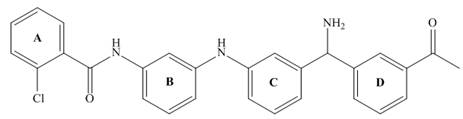
Consider the tetracyclic
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Viscosity of a liquid related to the activation energy.
Vibrational contributions to internal energy and heat capacity1) are temperature independent2) are temperature dependent
The approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.
Chapter 16 Solutions
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
Ch. 16.1 - Prob. 1PCh. 16.2 - Prob. 2PCh. 16.3 - Prob. 3PCh. 16.4 - Prob. 4PCh. 16.5 - Prob. 5PCh. 16.5 - Prob. 6PCh. 16.5 - Prob. 9PCh. 16.5 - Problem 18.9 Draw the product of each reaction
a....Ch. 16.5 - Prob. 11PCh. 16.5 - Prob. 12P
Ch. 16.5 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Problem 18.14 Draw all resonance structures for...Ch. 16.6 - Problem 18.15 Classify each substituent as...Ch. 16.7 - Prob. 17PCh. 16.9 - Prob. 22PCh. 16.10 - Problem 18.20 Draw the products of each...Ch. 16.10 - Prob. 24PCh. 16.11 - Problem 18.22 Draw the products formed when each...Ch. 16.12 - Problem 18.23 Devise a synthesis of each compound...Ch. 16.13 - Problem 18.24 Draw the products of each...Ch. 16.13 - Problem 18.25 Draw a stepwise mechanism for the...Ch. 16.13 - Problem 18.26 Draw the products of each...Ch. 16.13 - Prob. 30PCh. 16 - Prob. 37PCh. 16 - 18.35 What is the major product formed by an...Ch. 16 - 18.36 Draw the products formed when phenol is...Ch. 16 - Problem 18.37 Draw the products formed when each...Ch. 16 - 18.38 Draw the products of each reaction.
a. d....Ch. 16 - 18.39 What products are formed when benzene is...Ch. 16 - Prob. 49PCh. 16 - 18.47 For each of the following substituted...Ch. 16 - 18.48 Consider the tetracyclic aromatic compound...Ch. 16 - 18.49 For each N-substituted benzene, predict...Ch. 16 - Prob. 54PCh. 16 - 18.51 Using resonance structures, explain why a...Ch. 16 - Prob. 56PCh. 16 - 18.53 Rank the aryl halides in each group in order...Ch. 16 - Prob. 64PCh. 16 - Prob. 65PCh. 16 - Prob. 66PCh. 16 - 18.63 Synthesize each compound from benzene and...Ch. 16 - Problem 18.64 Synthesize each compound from...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain the meaning of: the electron partition function is equal to the degeneracy of the ground state.arrow_forward28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forwardAt 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forward
- Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardDraw the skeletal structure of the alkane 4-ethyl-2, 2, 5, 5- tetramethylnonane. How many primary, secondary, tertiary, and quantenary carbons does it have?arrow_forward
- Electronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY