
(a)
To explain: The reason why oxygen consumption is a good measure of
Introduction:
Amount of oxygen consumed is the measurement of activity of two stages of cellular respiration. These stages are: Citric acid cycle or TCA cycle which is an aerobic process, and glycolysis which is an anaerobic process. Oxaloacetate (OAA) and malate are the important part of citric acid cycle. In the given scenario sample of pigeon breast muscles were used to determine the oxygen consumption.
(a)
Explanation of Solution
Explanation:
The amount of oxygen consumed serves as a good measurement of cellular respiration because the reactions taking place in the pigeon breast muscles use the good amount of oxygen to perform the cellular actions. The consumption of oxygen by the cells depends on the rate of the activity of the cells.
If the oxygen is being consumed on a constant rate then the metabolic conditions of the cells are normal but abnormality in the metabolic conditions of the cells causes irregular consumption of oxygen.
(b)
To explain: The reason of some oxygen consumed by supplemented muscle tissues (sample 1).
Introduction:
The plants are capable in deriving the source from the direct sunlight, but other organisms such as human being or birds are not capable in obtaining energy from the sunlight. To generate energy the cells of such organisms perform the oxidation of glucose through various cycles.
(b)
Explanation of Solution
Explanation:
The sample obtained from the muscle tissues contains some amount of residual glucose. Residual glucose refers to the amount of glucose left over after several processes such as evaporation, filtration or distillation.
The residual glucose can initiate the oxidative phosphorylation by the help of other sugars and glycerol obtained from fats. Therefore the glucose residue is responsible for the oxygen consumption in the cells.
(c)
To determine: After examining the results of sample 2 and 3, whether 1- phosphoglycerol and citrate can be determined as the substrates for cellular respiration.
Introduction:
Oxygen is the prime requirement of the cells to perform the cellular respiration. Cellular respiration is performed by the cells to obtain energy. Respiration in the cells takes place in various steps in the form of cycles: glycolysis, citric acid cycle, and oxidative phosphorylation.
(c)
Explanation of Solution
Explanation:
1-Phosphoglycerol is an intermediate of glycolysis cycle. It is obtained from the reduction of dihydroxy acetone phosphate. Citrate is an intermediate of citric acid cycle. It is the first product of the cycle obtained from the combination of oxaloacetate and acetyl CoA.
Addition of these substances in the cycle can increase the amount of oxygen consumption in the cellular respiration. So, these substances can be considered as the substrates of the cellular respiration.
(d)
To determine: Whether citrate is catalytic for the
Introduction:
Citrate is an intermediate of citric acid cycle and addition of citrate increases the oxygen consumption. In the citric acid cycle when citrate converts into α-ketoglutarate, various types of reactions take place, as dehydration, rehydration, and oxidative decarboxylation. In these
(d)
Explanation of Solution
Explanation:
Experiment 1 depicts that citrate is consuming more oxygen than expected. Citrate follows the characteristic of a catalyst that it takes part in the reaction more than once.
Experiment 2 depicts that with the addition of citrate and 1-phosphoglycerol the consumption of oxygen increases multi folds.
Data in the table shows that excess oxygen consumed by citrate is 89 µL and oxygen consumed by 1-phosphoglycerol is 415 µL, the additional product of both gives 504 µL.
When 0.15 mL of 0.2 M citrate and 0.3 mL of 0.2 M 1-phosphoglycerol are added then oxygen consumption is 1043 µL.
The difference between 1043 µL and 504 µL is 539 µL. This huge difference in the oxygen consumption is because of the citrate activity. The large difference in the amount of oxygen consumed shows the catalytic activity of citrate.
Tabular representation: The Table.1 shows the consumption of excess oxygen.
Table 1: The consumption of excess oxygen.
| Sample | Added Substrate | Absorbed amount of O2 in
|
Excess amount of O2 consumed in
|
| 1 | no addition of extra substrate | 342 | 0 |
| 2 | 0.3 mL of 0.2 M 1-phosphoglycerol is added. | 757 | 415 |
| 3 | 0.15 mL of 0.2 M citrate added | 431 | 89 |
| 4 | 0.15 mL of 0.2 M citrate and 0.3 mL of 0.2 M 1-phosphoglycerol are added. | 1385 | 1043 |
(e)
To discuss: Whether the citrate was regenerated in the whole process so the reactions occurred in cyclic manner rather than linear pathway.
Introduction:
In the citric acid cycle when citrate converts into α-ketoglutarate, various types of reactions take place, as dehydration, rehydration, and oxidative decarboxylation. In these chemical reactions some byproducts are obtained, the by-products contain the atoms or functional group broken from the actual reactant. This results in the loss of some amount of the substrates.
(e)
Explanation of Solution
Explanation:
The data table shows that the pathway consumes citrate. If citrate acts as the catalyst it must be regenerated because catalyst takes part in the pathways at multiple steps.
This pathway first consumes the citrate and then regenerates it. It is clear that the pathway is cyclic rather than linear, because the linear pathway consumes any substrate for once and cannot regenerate it.
(f)
To determine: The product obtained from the conversion of citrate in the given pathway and the reason behind the consumption of oxygen by the sample.
Introduction:
α-ketoglutarate dehydrogenase is inhibited by arsenate and succinate dehydrogenase is inhibited by malonate. α-ketoglutarate dehydrogenase inhibition causes failure in production of succinyl CoA and inhibition in succinate dehydrogenase causes failure of production of fumarate.
(f)
Explanation of Solution
Explanation:
Citrate converts into isocitrate which eventually converts into α-ketoglutarate. The inhibition in the α-ketoglutarate dehydrogenase enzyme activity restricts the cycle at α-ketoglutarate.
Oxygen is consumed by the sample for the reoxidation of NADH. NADH is produced at three steps of citric acid cycle. First, it is produced during the conversion of isocitrate into α-ketoglutarate, then it is produced during conversion of α-ketoglutarate to succinyl CoA, and the third step of NADH production is the conversion of malate into oxaloacetate.
As arsenate inhibits the activity of α-ketoglutarate dehydrogenase the conversion of α-ketoglutarate does not occur. Oxygen is consumed for the reoxidation of NADH at the step of conversion of isocitrate into α-ketoglutarate.
(g)
To determine: The order of intermediates obtained in the citric acid cycle on the basis of data given and to compare it with the citric acid cycle.
Introduction:
Addition of oxaloacetate stimulates the consumption of oxygen many times. Oxaloacetate is the important part of citric acid cycle. Addition of these substances stimulates the citric acid cycle hence consumption of oxygen increases.
(g)
Explanation of Solution
Explanation:
Based on the data given, the correct order of intermediate obtained in the citric acid cycle is depicted in the Fig.1 “The cyclic order of intermediate obtained”.
The cycle of intermediates obtained from the data is different from the original citric acid cycle, as the cycle shown in Fig.1 does not contain acetyl CoA, cis-aconitase, isocitrate, and succinyl CoA.
Pictorial representation: Fig.1 shows the cycle of the intermediates obtained.
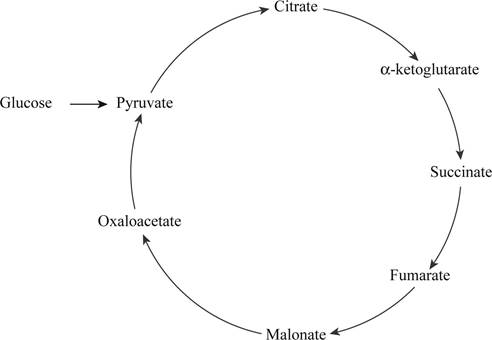
Fig.1: The cyclic order of intermediate obtained.
(h)
To explain: The importance of quantitative conversion of citrate to α-ketoglutarate.
Introduction:
In the citric acid cycle when citrate converts into α-ketoglutarate, various types of reactions take place, as dehydration, rehydration, and oxidative decarboxylation. In these chemical reactions some byproducts are obtained, the by-products contain the atoms or functional group broken from the actual reactant. This results in the loss of some amount of the substrates.
(h)
Explanation of Solution
Explanation:
Some citric acid cycle intermediates get involve with the other pathways in the form of catalyst or the reactants, thus it losses some amount of important atoms and molecules.
Quantitative conversion of citrate into α-ketoglutarate is important to ongoing pathway not to get clubbed or complexed with some other pathways.
Want to see more full solutions like this?
Chapter 16 Solutions
EBK LEHNINGER PRINCIPLES OF BIOCHEMISTR
- MISSED THIS? Read Section 19.9 (Pages 878-881); Watch IWE 19.10 Consider the following reaction: CH3OH(g) CO(g) + 2H2(g) (Note that AG,CH3OH(g) = -162.3 kJ/mol and AG,co(g)=-137.2 kJ/mol.) Part A Calculate AG for this reaction at 25 °C under the following conditions: PCH₂OH Pco PH2 0.815 atm = 0.140 atm 0.170 atm Express your answer in kilojoules to three significant figures. Ο ΑΣΦ AG = -150 Submit Previous Answers Request Answer □? kJ × Incorrect; Try Again; 2 attempts remaining Calculate the free energy change under nonstandard conditions (AGrxn) by using the following relationship: AGrxn = AGrxn + RTInQ, AGxn+RTInQ, where AGxn is the standard free energy change, R is the ideal gas constant, T is the temperature in kelvins, a is the reaction quotient. Provide Feedback Next >arrow_forwardIdentify and provide a brief explanation of Gas Chromatography (GC) within the context of chemical analysis of food. Incorporate the specific application name, provide a concise overview of sample preparation methods, outline instrumental parameters and conditions ultilized, and summarise the outcomes and findings achieved through this analytical approach.arrow_forwardIdentify and provide a concise explanation of the concept of signal-to-noise ratio (SNR) in the context of chemical analysis. Provide specific examples.arrow_forward
- Identify and provide a concise explanation of a specific analytical instrument capable of detecting and quantifying trace compounds in food samples. Emphasise the instrumental capabilities relevant to trace compound analysis in the nominated food. Include the specific application name (eg: identification and quantification of mercury in salmon), outline a brief description of sample preparation procedures, and provide a summary of the obtained results from the analytical process.arrow_forwardIdentify and provide an explanation of what 'Seperation Science' is. Also describe its importance with the respect to the chemical analysis of food. Provide specific examples.arrow_forward5. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn. H3C CH3arrow_forward
- State the name and condensed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardState the name and condensed formula of the isothiazole obtained by reacting acetylacetone and thiosemicarbazide.arrow_forwardProvide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forward
- Given a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forwardThe molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





