
Concept explainers
Show how each of the following compounds can be synthesized from cyclopentanol and any necessary organic or inorganic reagents. In many cases the desired compound can be made from one prepared in an earlier part of the problem.
trans-
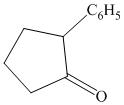
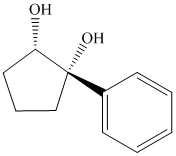
Interpretation:
Each of the given product is to be synthesized from cyclopentanol and necessary organic or inorganic reagents.
Concept introduction:
Alcohols can be prepared from a variety of reagents.
Reaction of Grignard reagents with carbonyl compounds produces the corresponding alcohols.
Grignard reagents also react with oxiranes to produce alcohols.
The allylic and benzylic carbon atoms are selectively brominated using NBS reagent.
Alcohols undergo dehydration in acidic medium, producing alkenes. These alkenes can be converted to diols using osmium tetra oxide.
Answer to Problem 23P
Solution:
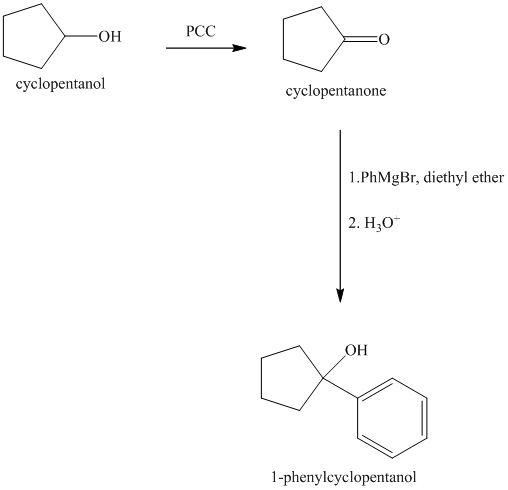
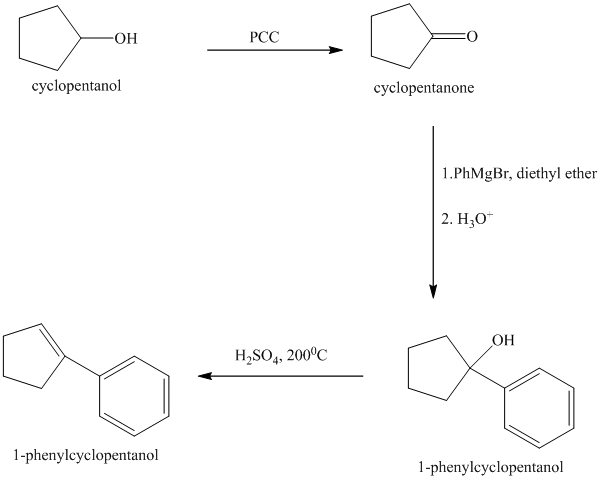
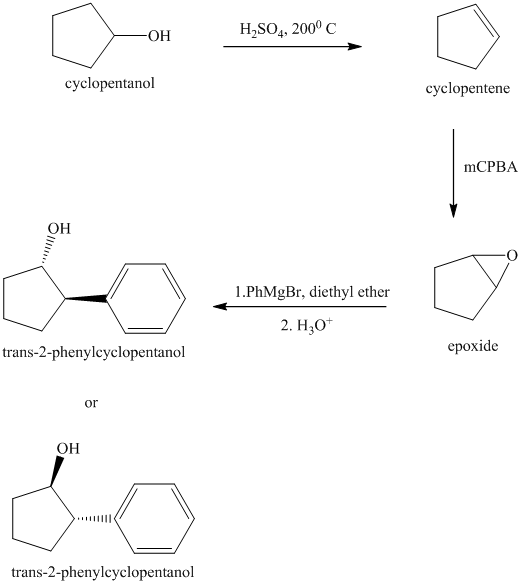
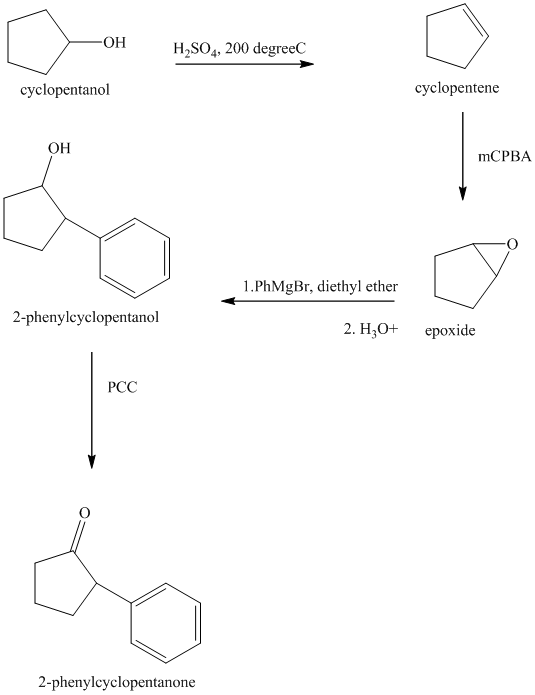
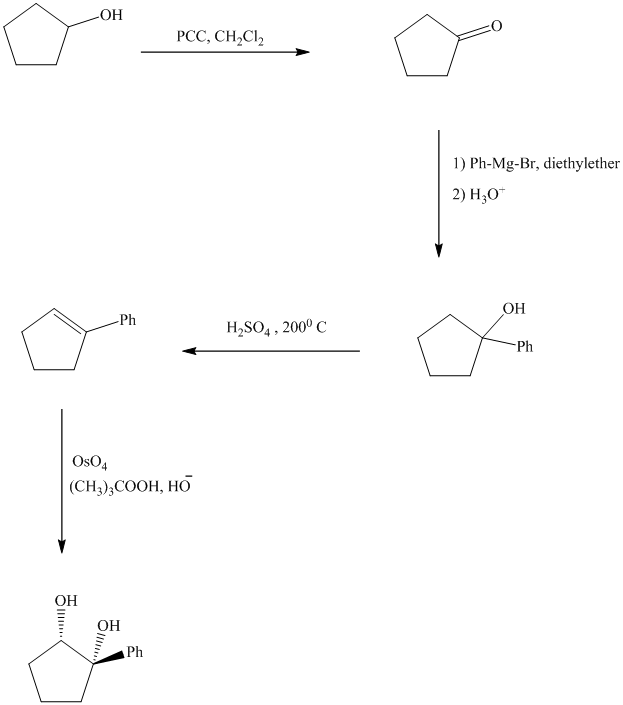
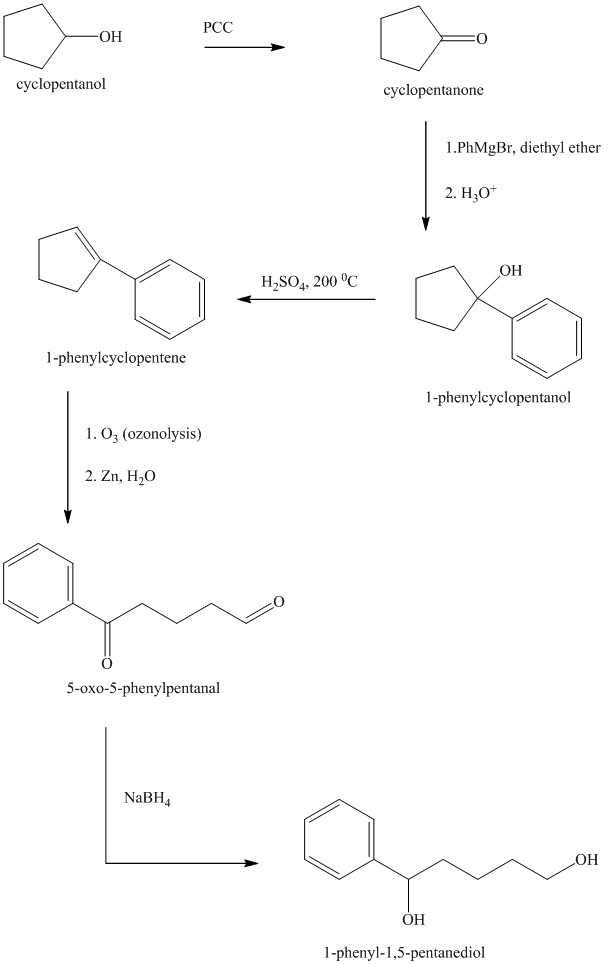
Explanation of Solution
Synthesis of
The structure for cyclopentanol and
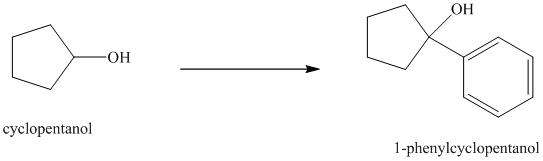
In

Synthesis of
The structure for cyclopentanol and
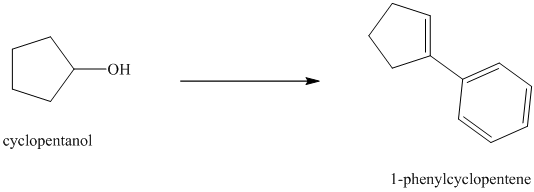
In
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
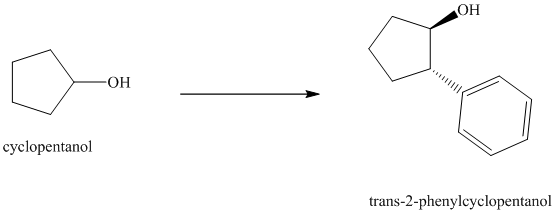
In
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
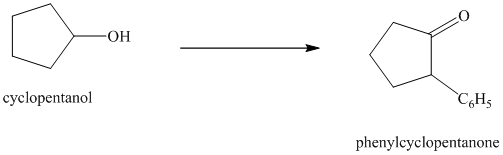
In
The sequence of reactions starting from cyclopentanol to yield the final given product
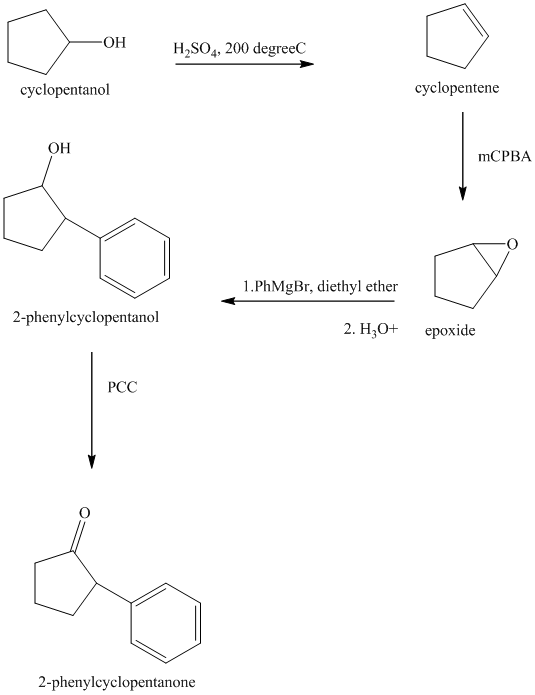
Synthesis of the given diol from cyclopentanol.
The structure for the given diol is as follows:
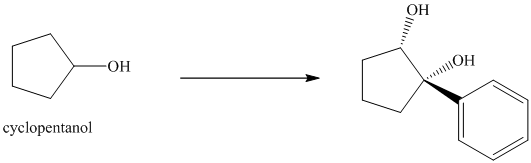
In the given diol, one phenyl ring and one hydroxyl group are attached to the same carbon of cyclopentane ring. The other hydroxyl group is attached to C2 position of cyclopentane ring.
Oxidation of the cyclopentanol will produce cyclopentanone. Reaction of this cyclopentanone with phenyl magnesium bromide will form a tertiary alcohol. Acid catalyzed dehydration of this tertiary alcohol will produce
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
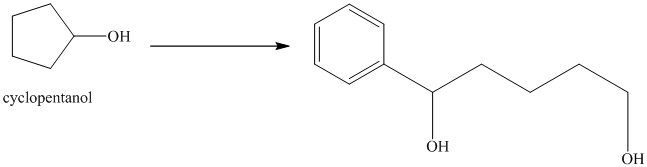
Cyclopentanol, when undergoes oxidation in presence of a mild oxidizing agent such as pyridinium chlorochromate in dichlormethane, the hydroxyl group turns to a carbonyl group and forms cyclopentanone. Cyclopentanone, when treated with Grignard reagent (PhMgBr) in the presence of diethyl ether with acidic workup, forms
The sequence of reactions is shown below.

Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning




