
Concept explainers
(a)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
The second resonance structure for given species and its hybrid are,
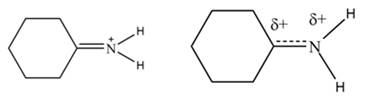
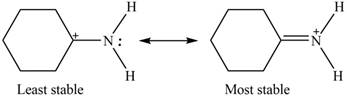
The increasing order of stability for the given resonance structure is,
Explanation of Solution
The given species is shown below.
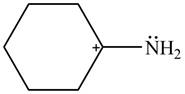
Figure 1
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
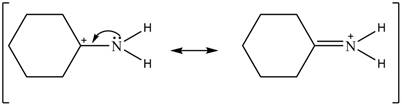
Figure 2
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
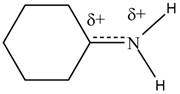
Figure 3
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
The Figure 2 shows that the left side structure does not contain double bond. Thus, the stability of left side structure is less than the right side structure.
Therefore, the increasing order of stability for the given resonance structure is shown below.
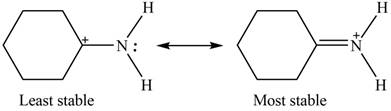
Figure 4
The second resonance structure for given species and its hybrid is shown in Figure 2 and 3. The increasing order of stability for the given resonance structure is shown in Figure 4.
(b)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
The second resonance structure for given species and its hybrid are,
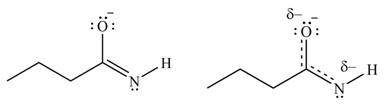
The increasing order of stability for the given resonance structure is,
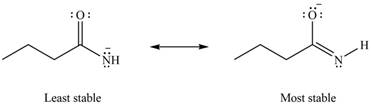
Explanation of Solution
The given species is shown below.
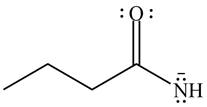
Figure 5
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
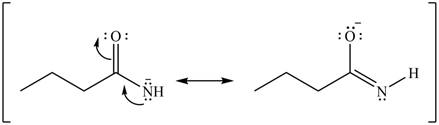
Figure 6
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
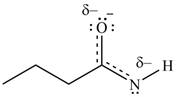
Figure 7
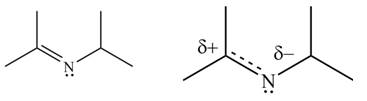
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure in which negative charge is on more electronegative atom. The electronegativity of oxygen is more than nitrogen.
The Figure 6 shows that in the left side structure, negative charge is on nitrogen atom, whereas in right side structure, negative charge is on oxygen atom.. Thus, the stability of left side structure is less than the right side structure.
Therefore, the increasing order of stability for the given resonance structure is shown below.
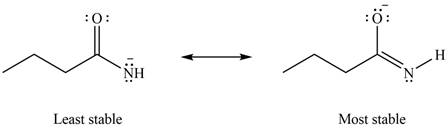
Figure 8
The second resonance structure for given species and its hybrid is shown in Figure 6 and 7. The increasing order of stability for the given resonance structure is shown in Figure 8.
(c)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
The second resonance structure for given carbocation and its hybrid are,
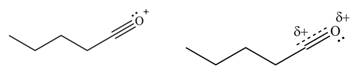
The increasing order of stability for the given resonance structure is,
Explanation of Solution
The given species is shown below.
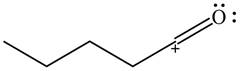
Figure 9
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
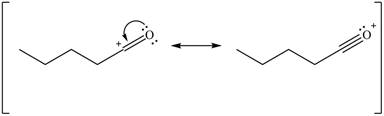
Figure 10
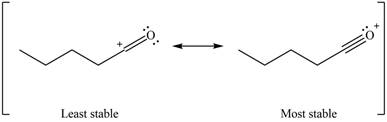
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
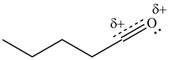
Figure 11
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
Therefore, the increasing order of stability for the given resonance structure is shown below.

Figure 12
The second resonance structure for given carbocation and its hybrid is shown in Figure 10 and 11. The increasing order of stability for the given resonance structure is shown in Figure 12.
(d)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.7P
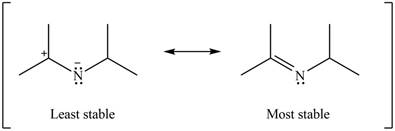
The second resonance structure for given carbocation and its hybrid are,
The increasing order of stability for the given resonance structure is,
Explanation of Solution
The given species is shown below.
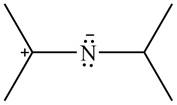
Figure 13
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
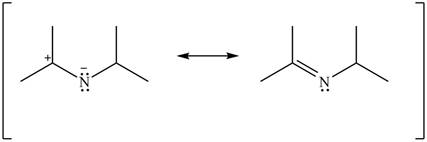
Figure 14
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
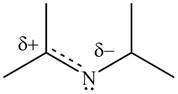
Figure 15
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
The given Figure 2 shows that the left side structure does not contain double bond. Thus, the stability of left side structure is less than the right side structure.
Therefore, the increasing order of stability for the given resonance structure is shown below.

Figure 16
The second resonance structure for given carbocation and its hybrid is shown in Figure 14 and 15. The increasing order of stability for the given resonance structure is shown in Figure 16.
Want to see more full solutions like this?
Chapter 16 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
- 1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following compounds. Please show your work. (8) SF2, CH,OH, C₂H₂ b) Based on your answers given above, list the compounds in order of their Boiling Point from low to high. (8)arrow_forward19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forward
- Q4: Write organic product(s) of the following reactions and show the curved-arrow mechanism of the reactions. Br MeOH OSO2CH3 MeOHarrow_forwardProvide the correct IUPAC name for the compound shown here. Reset cis- 5- trans- ☑ 4-6- 2- 1- 3- di iso tert- tri cyclo sec- oct but hept prop hex pent yl yne ene anearrow_forwardQ6: Predict the major product(s) for the following reactions. Note the mechanism (SN1, SN2, E1 or E2) the reaction proceeds through. If no reaction takes place, indicate why. Pay attention to stereochemistry. NaCN DMF Br σ Ilm... Br H Br H H NaCN CH3OH KOtBu tBuOH NaBr H₂O LDA Et2O (CH3)2CHOH KCN DMSO NaOH H₂O, A LDA LDA Systemarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





