
Concept explainers
(a)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.60P
The products formed by the given reaction are,
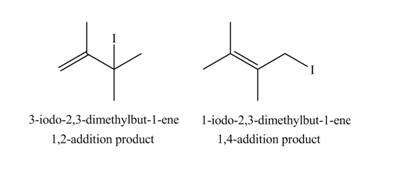
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov addition of
The given reaction is shown below.
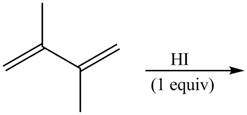
Figure 1
The given diene is a conjugated diene. The attack of
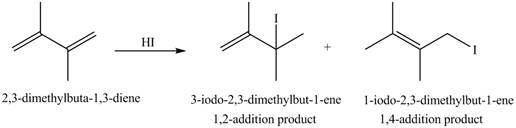
Figure 2
Therefore, the products formed by the given reaction are
The products formed by the given reaction is
(b)
Interpretation: The Diels-Alder product formed by the given reaction including stereochemistry is to be drawn.
Concept introduction: A
Answer to Problem 16.60P
The Diels-Alder product formed by the given reaction is,
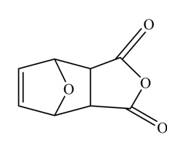
Explanation of Solution
The given reaction is shown below.
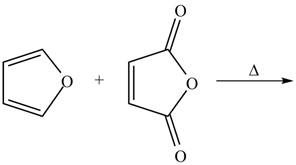
Figure 3
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. They have syn stereochemistry. The stereochemistry of dienophile in endo isomer is same as that of bridging ring system, whereas in exo isomer, it is in opposite.
Thus, the Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 4.
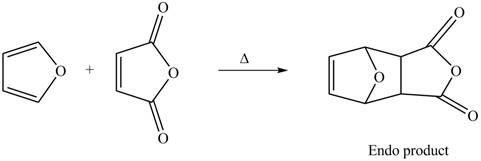
Figure 4
The Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 4.
(c)
Interpretation: The Diels-Alder product formed by the given reaction including stereochemistry is to be drawn.
Concept introduction: A chemical reaction that involves
Answer to Problem 16.60P
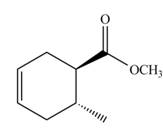
The Diels-Alder product formed by the given reaction including stereochemistry is,
Explanation of Solution
The given reaction is shown below.
![]()
Figure 5
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. They have syn stereochemistry.
Thus, the Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 4.
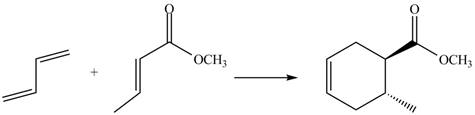
Figure 6
The Diels-Alder product formed by the given reaction including stereochemistry is shown in Figure 6.
(d)
Interpretation: The product formed by the reaction of given diene with one equivalent of
Concept introduction: Diene is a hydrocarbon that contains two
Answer to Problem 16.60P
The products formed by the given reaction are,
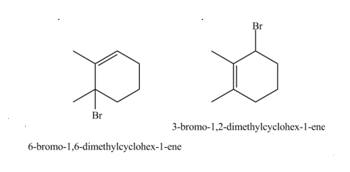
Explanation of Solution
Conjugated dienes undergoes electrophilic addition to gives a mixture of products that is
Markovnikov addition of
The given reaction is shown below.
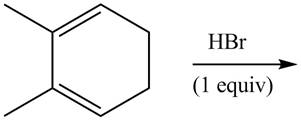
Figure 7
The given diene is a conjugated diene. The attack of
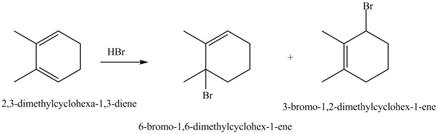
Figure 8
Therefore, the products formed by the given reaction is
The products formed by the given reaction is
Want to see more full solutions like this?
Chapter 16 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Identify as E1 or E2 and write the mechanism.arrow_forwardIdentify if their reaction is most likely SN1 or SN2 mechanism.arrow_forwardDraw the products formed when the following alkene is treated with 03 followed by Zn, H₂O. Be sure to answer all parts. draw structure ... smaller molar mass product draw structure ... larger molar mass productarrow_forward
- Identify as SN1 or SN2 and write the mechanism.arrow_forwardComplete the reaction. Not the mechanism.arrow_forwardDraw the mechanism using the arrows on conventions, including all formal charges and correct arrows. If stereochemical distinction can be made they should be included in the structure of the products.arrow_forward
- Draw the epoxide formed when the following alkene is treated with mCPBA. Click the "draw structure" button to launch the drawing utility. draw structure ...arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check CF3 (Choose one) OH (Choose one) H (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardIdentifying electron-donating and electron-withdrawing effects For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density CF3 O donating O donating O electron-rich O withdrawing withdrawing O no inductive effects O no resonance effects O electron-deficient O similar to benzene OCH3 Explanation Check O donating O donating ○ withdrawing withdrawing O no inductive effects no resonance effects electron-rich electron-deficient O similar to benzene Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





