
(a)
Interpretation:
For the given compound, both
Concept introduction:
Each chemically distinct type of hydrogen atom in a molecule gives rise to an independent signal in a proton NMR spectrum. In a
The
Answer to Problem 16.79P
For the given compound, both
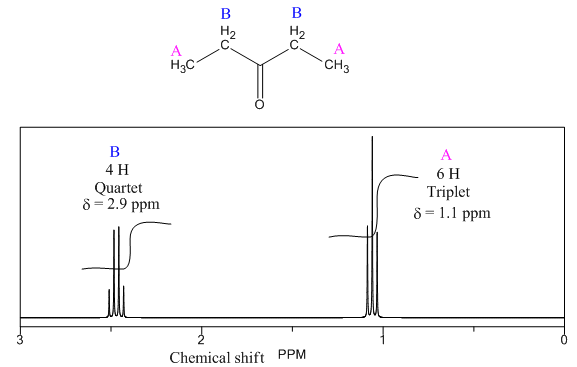
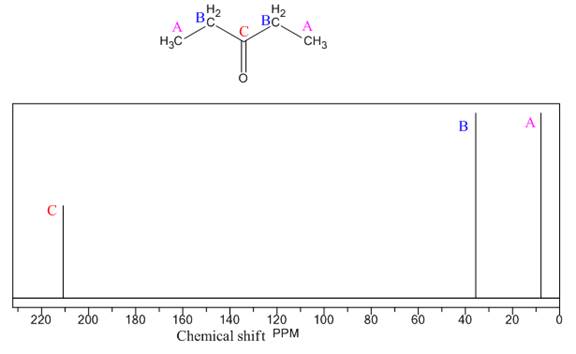
Explanation of Solution
The given compound is
There are two types of chemically different H atoms and three types of C atoms in the above compound. Thus, the

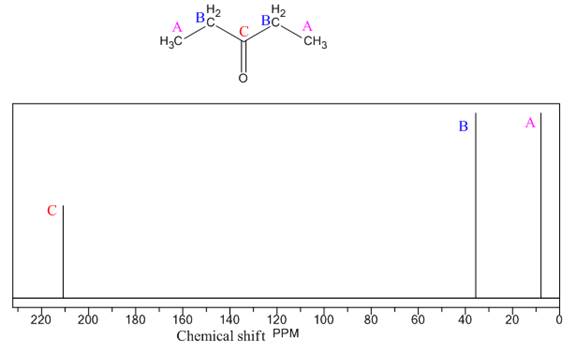
The
(b)
Interpretation:
For the given compound, both
Concept introduction:
Each chemically distinct type of hydrogen atom in a molecule gives rise to an independent signal in a proton NMR spectrum. In a
The
Answer to Problem 16.79P
For the given compound, both
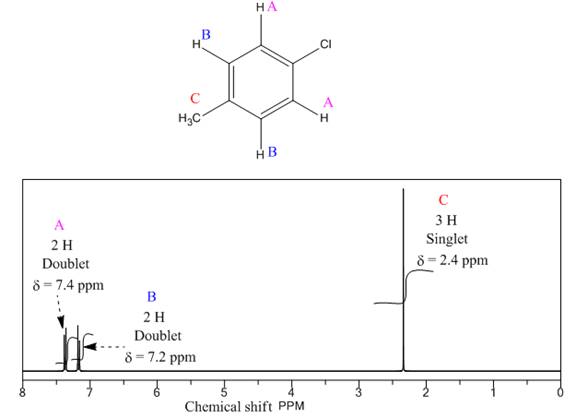
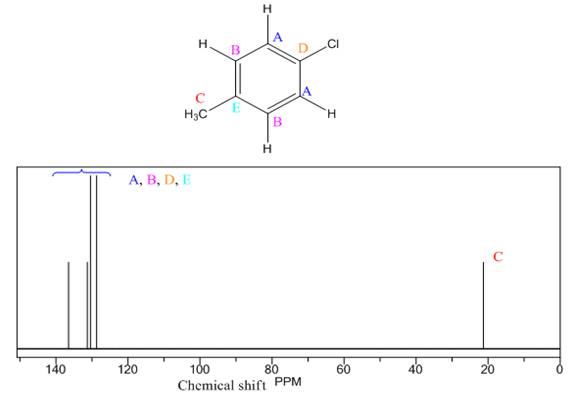
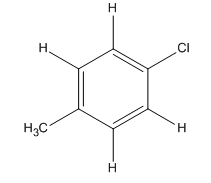
Explanation of Solution
The given compound is
There are three types of chemically different H atoms and five types of C atoms in the above compound. Thus, the
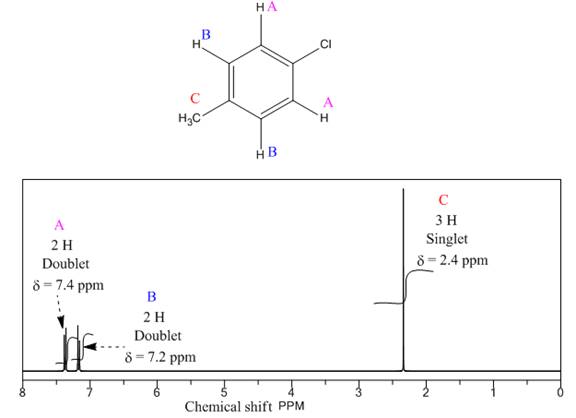
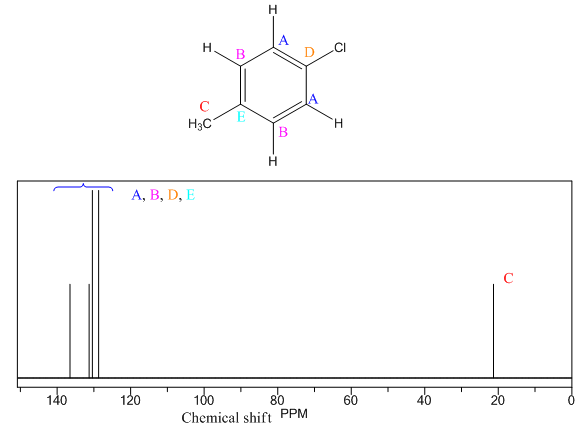
The
(c)
Interpretation:
For the given compound, both
Concept introduction:
Each chemically distinct type of hydrogen atom in a molecule gives rise to an independent signal in a proton NMR spectrum. In a
The
Answer to Problem 16.79P
For the given compound, both
Explanation of Solution
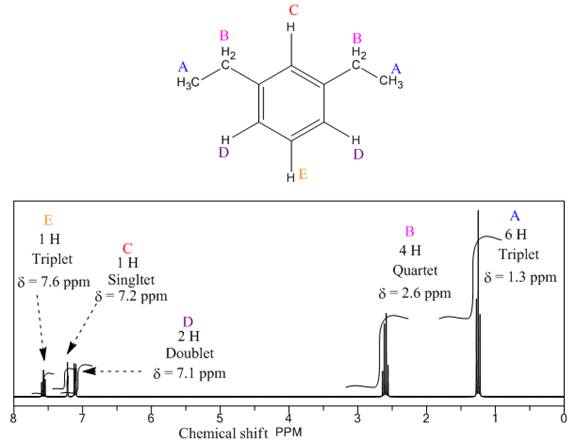
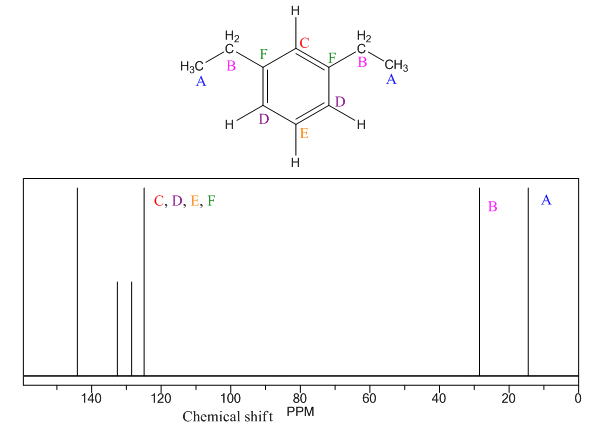
The given compound is
There are five types of chemically different H atoms and six types of C atoms in the above compound. Thus, the
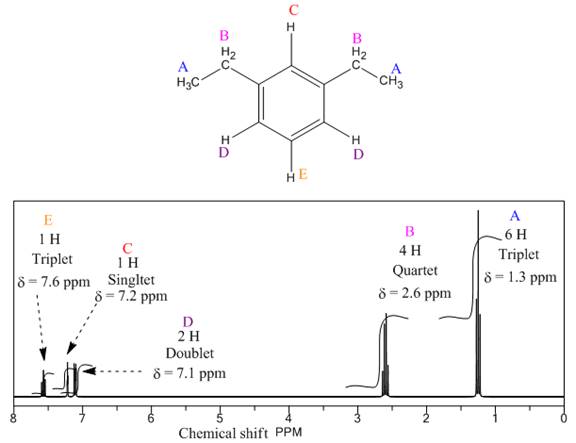

The
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- A certain inorganic cation has an electrophoretic mobility of 5.27 x 10-4 cm2s-1V-1. The same ion has a diffusion coefficient of 9.5 x 10-6cm2s-1. If this ion is separated from cations by CZE with a 75cm capillary, what is the expected plate count, N, at an applied voltage of 15.0kV? Under these separation conditions, the electroosmotic flow rate was 0.85mm s-1 toward the cathode. If the detector was 50.0cm from the injection end of the capillary, how long would it take in minutes for the analyte cation to reach the detector after the field was applied?arrow_forward2.arrow_forwardPlease solve for the following Electrochemistry that occursarrow_forward
- Commercial bleach contains either chlorine or oxygen as an active ingredient. A commercial oxygenated bleach is much safer to handle and less likely to ruin your clothes. It is possible to determine the amount of active ingredient in an oxygenated bleach product by performing a redox titration. The balance reaction for such a titration is: 6H+ +5H2O2 +2MnO4- à 5O2 + 2Mn2+ + 8H2O If you performed the following procedure: “First, dilute the Seventh Generation Non-Chlorine Bleach by pipetting 10 mL of bleach in a 100 mL volumetric flask and filling the flask to the mark with distilled water. Next, pipet 10 mL of the diluted bleach solution into a 250 mL Erlenmeyer flask and add 20 mL of 1.0 M H2SO4 to the flask. This solution should be titrated with 0.0100 M KMnO4 solution.” It took 18.47mL of the KMnO4 to reach the endpoint on average. What was the concentration of H2O2 in the original bleach solution in weight % assuming the density of bleach is 1g/mL?arrow_forward10.arrow_forwardProper care of pH electrodes: Why can you not store a pH electrode in distilled water? What must you instead store it in? Why?arrow_forward
- Write the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 569 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! §arrow_forwardIdentify the amino acids by name. Illustrate a titration curve for this tetrapeptide indicating the pKa's for each ionizable groups and identify the pI for this tetrapeptide. please helparrow_forward↓ ina xSign x Sign X labs X Intro X Cop Xa chat X My Cx Grac X Laur x Laur xash learning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db2 +949 pts /3400 K Question 16 of 34 > © Macmillan Learning Draw the major E2 reaction product formed when cis-1-chloro-2-ethylcyclohexane (shown) reacts with hydroxide ion in DMSO. H CH2CH3 H H HO- H H H Cl DMSO H H C Select Draw Templates More C H 0 2 Erasearrow_forward
- A common buffer for stabilizing antibodies is 100 mM Histidine at pH 7.0. Describe the preparation of this buffer beginning with L-Histidine monohydrochloride monohydrate and 1 M NaOH. Be certain to show the buffering reaction that includes the conjugate acid and base.arrow_forwardFina x | Sign X Sign X lab: X Intro X Cop) X a chat x My x Grad xLaur x Laur x a sheg X S Shoj XS SHE X acmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2- 5 © Macmillan Learning Organic Chemistry Maxwell presented by Macmillan Learning For the dehydrohalogenation (E2) reaction shown, draw the Zaitsev product, showing the stereochemistry clearly. H H KOH Br EtOH Heat Select Draw Templates More Erase // C H Q Search hp Q2 Q Δ קו Resouarrow_forwardIs the structural form shown possible given the pKa constraints of the side chains?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





