Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
11th Edition
ISBN: 9781305081055
Author: Bettelheim, Frederick A.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 16, Problem 16.56P
Interpretation Introduction
Interpretation:
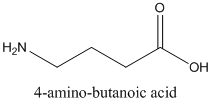
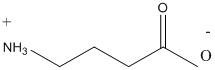
Following are the two structural formulas for 4-amino-butanoic acid, a neurotransmitter. The better structural formula for the above molecule is (A) or (B).
Concept introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Is this aromatic?
CHEM2323
E
Tt
PS CH03
Draw and name all monobromo derivatives of pentane, C5H11Br.
Problem 3-33
Name:
Draw structures for the following:
(a) 2-Methylheptane
(d) 2,4,4-Trimethylheptane
Problem 3-35
(b) 4-Ethyl-2,2-dimethylhexane
(e) 3,3-Diethyl-2,5-dimethylnonane
(c) 4-Ethyl-3,4-dimethyloctane
2
(f) 4-Isopropyl-3-methylheptane
KNIE>
Problem 3-42
Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond:
(a) Draw a Newman projection of the most stable
conformation.
(b) Draw a Newman projection of the least stable
conformation.
Problem 3-44
Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane.
Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2-
dibromoethane.
Problem 3-45
Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole
moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the
actual conformation of the molecule?
Chapter 16 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
Ch. 16.1 - Problem 16-1 How many hydrogen atoms does...Ch. 16.2 - Problem 16-2 Write a structural formula for each...Ch. 16.2 - Prob. 16.3PCh. 16.4 - Problem 16-4 Select the stronger base from each...Ch. 16.5 - Prob. 16.5PCh. 16 - 16-6 Answer true or false. te/7-Butylamine is a 3°...Ch. 16 - Prob. 16.7PCh. 16 - Prob. 16.8PCh. 16 - 16-9 In what way are pyridine and pyrimidine...Ch. 16 - Prob. 16.10P
Ch. 16 - Prob. 16.11PCh. 16 - Prob. 16.12PCh. 16 - 16-13 Classify each amino group as primary,...Ch. 16 - Prob. 16.14PCh. 16 - 16-15 There are eight primary amines with the...Ch. 16 - Prob. 16.16PCh. 16 - 16-17 Propylamine (bp 48°C), ethylmethylamine (bp...Ch. 16 - 16-18 Account for the fact that 1-butanamine (bp...Ch. 16 - 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol...Ch. 16 - Prob. 16.20PCh. 16 - Prob. 16.21PCh. 16 - Prob. 16.22PCh. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25PCh. 16 - 16-26 The p/fb of amphetamine is approximately 3.2...Ch. 16 - 16-27 Guanidine, p/Ca 13.6, is a very strong base,...Ch. 16 - 16-28 Following is the structural formula of...Ch. 16 - Prob. 16.29PCh. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - 16*32 Many tumors of the breast are correlated...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16-35 (Chemical Connections 16B ) What is an...Ch. 16 - Prob. 16.36PCh. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - Prob. 16.41PCh. 16 - Prob. 16.42PCh. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - Prob. 16.45PCh. 16 - 16-46 Arrange these three compounds in order of...Ch. 16 - Prob. 16.47PCh. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - Prob. 16.53PCh. 16 - 16-54 Several poisonous plants, including Atropa...Ch. 16 - Prob. 16.55PCh. 16 - Prob. 16.56PCh. 16 - Prob. 16.57PCh. 16 - 16-58 Following is a structural formula of...Ch. 16 - Prob. 16.59P
Knowledge Booster
Similar questions
- Gas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forward
- Nonearrow_forward4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co