
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.33SP
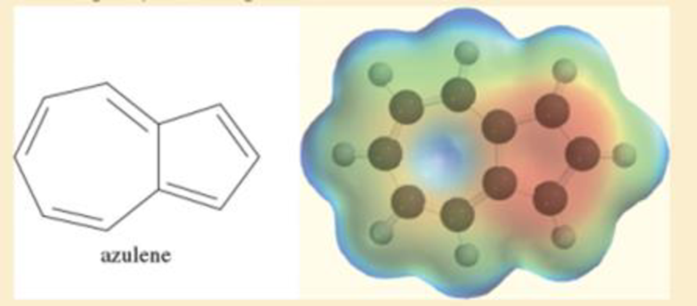
Azulene is a deep-blue hydrocarbon with resonance energy of 205 kJ/mol (49 kcal/mol). Azulene has ten pi electrons, so it might be considered as one large
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The structure of compound 1,3,5-trimethylbenzene (mesitylene) is given below.
How many signals would you expect to find in the 'H NMR spectrum of 1,3,5-trimethylbenzene (mesitylene)?
Check
×
1
How many signals do you expect in the 'H NMR spectrum for this molecule?
CI CI
Cl
Write the answer in the table below.
Also, in each of the drawing areas below is a copy of the molecule, with H atoms shown. In each copy, one of the H atoms is highlighted red. Highlight in red all
other H atoms that would contribute to the same signal as the H already highlighted red.
Note for advanced students: Remember, a multiplet is considered one signal in the 'H NMR spectrum.
1
Number of signals in the 'H NMR spectrum.
☐
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same
signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional H atoms to highlight in
top molecule
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the
same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at…
wrtie the balanced equation and find the E° when the following half- reactions are combined
Zn2+(aq) + 2e---> Zn(s) E°= -0.763V
Ag+(aq) + e---> Ag (s) E°=+0.799V
Chapter 16 Solutions
Organic Chemistry (9th Edition)
Ch. 16.2 - Prob. 16.1PCh. 16.2 - Prob. 16.2PCh. 16.2 - a. Draw the resonance forms of benzene,...Ch. 16.2 - Show the product of the Diels-Alder dimerization...Ch. 16.4 - Prob. 16.5PCh. 16.6 - Make a model of cyclooctatetraene in the tub...Ch. 16.6 - Prob. 16.7PCh. 16.6 - Prob. 16.8PCh. 16.7 - Prob. 16.9PCh. 16.8A - a. Draw the molecular orbitals for the...
Ch. 16.8A - Repeat Problem16-10 for the cyclopentadienyl ions....Ch. 16.8C - Explain why each compound or ion should be...Ch. 16.8C - The following hydrocarbon has an unusually large...Ch. 16.8C - Prob. 16.14PCh. 16.8C - Prob. 16.15PCh. 16.9B - Prob. 16.16PCh. 16.9C - Show which of the nitrogen atoms in purine are...Ch. 16.9C - The proton NMR spectrum of 2-pyridone gives the...Ch. 16.9D - Prob. 16.19PCh. 16.9D - Prob. 16.20PCh. 16.10 - Prob. 16.21PCh. 16.12 - Ciprofloxacin is a member of the fluoroquinolone...Ch. 16.13 - Draw and name all the chlorinated benzenes having...Ch. 16.13 - Name the following compounds:Ch. 16.15 - The UV spectrum of 1-phenylprop-2-en-1-ol shows an...Ch. 16 - Prob. 16.26SPCh. 16 - Name the following compounds:Ch. 16 - Draw and name all the methyl, dimethyl, and...Ch. 16 - Four pairs of compounds are shown. In each pair,...Ch. 16 - One of the following hydrocarbons is much more...Ch. 16 - In Kekuls time cyclohexane was unknown, and there...Ch. 16 - Prob. 16.32SPCh. 16 - Azulene is a deep-blue hydrocarbon with resonance...Ch. 16 - Prob. 16.34SPCh. 16 - Prob. 16.35SPCh. 16 - Prob. 16.36SPCh. 16 - Prob. 16.37SPCh. 16 - Prob. 16.38SPCh. 16 - Prob. 16.39SPCh. 16 - Biphenyl has the following structure. a. Is...Ch. 16 - Anions of hydrocarbons are rare, and dianions of...Ch. 16 - How would you convert the following compounds to...Ch. 16 - Prob. 16.43SPCh. 16 - Prob. 16.44SPCh. 16 - A student found an old bottle labeled thymol on...Ch. 16 - Prob. 16.46SPCh. 16 - Prob. 16.47SPCh. 16 - Prob. 16.48SPCh. 16 - The proton NMR chemical shifts of the hydrogens in...Ch. 16 - Prob. 16.50SPCh. 16 - NMR has been used to probe many molecular...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 'H NMR spectrum? Note: A multiplet is considered one signal. ☐arrow_forwardStudy this 'H NMR spectrum, and then answer the questions about it in the table below. Check 1.0- 0.5- 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 What unit symbol should be written on the horizontal axis? What is the chemical shift & of the doublet? If there is no doublet, just check the box instead. Give your answer to 2 significant digits. What is the chemical shift of the signal immediately upfield of the doublet? If there is no doublet, or no signal upfield of it, check the box instead. What is the chemical shift & of the least deshielded proton? If you can't tell without more information, check the box instead. 血 8 = ☐ There is no doublet. 8 = ☐ No such signal. 8 = 0 Need more information.arrow_forwardhow many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forward
- calculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forwardan adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forwardWhat are the advantages and/or disadvantages of using the MOHR titration method & AOEC method?arrow_forward
- Are there any alternative methods better than the MOHR titration to quantitatively determine salt in a sample?arrow_forwardhybridization of nitrogen of complex moleculesarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forward
- Why do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forwardWhat are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY