
Draw all reasonable resonance structures for each species.
a.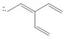 b.
b. c.
c.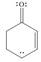 d.
d.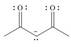 e.
e.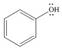 f.
f. 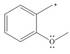
(a)
Interpretation: The possible resonance structure for the given species is to be drawn.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.32P
The possible resonance structure for the given species is,
Explanation of Solution
The method by which overall delocalization of electrons can be described in a particular molecule is known as resonance.
The given species is shown below.
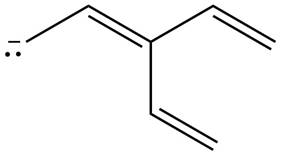
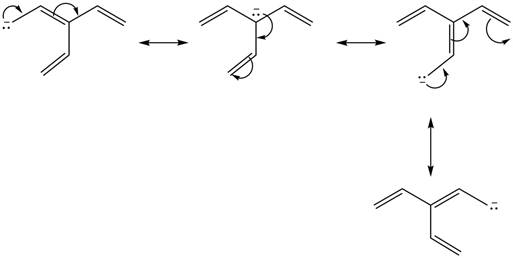
Figure 1
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
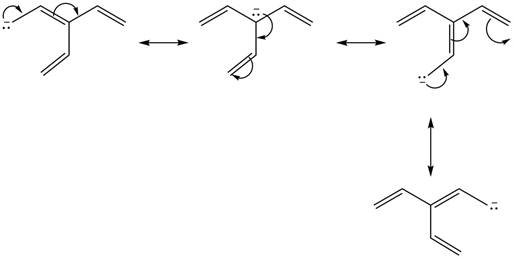
Figure 2
The possible resonance structure for the given species is shown in Figure 2.
(b)
Interpretation: The possible resonance structure for the given species is to be drawn.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.32P
The possible resonance structure for the given species is,
![]()
Explanation of Solution
The method by which overall delocalization of electrons can be described in a particular molecule is known as resonance. Carbocation shows resonance structures when double bond is present in conjugation with it.
The given species is shown below.
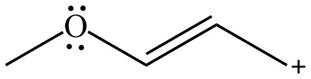
Figure 3
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
![]()
Figure 4
The possible resonance structure for the given species is shown in Figure 4.
(c)
Interpretation: The possible resonance structure for the given species is to be drawn.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.32P
The possible resonance structure for the given species is,
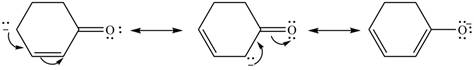
Explanation of Solution
The method by which overall delocalization of electrons can be described in a particular molecule is known as resonance.
The given species is shown below.
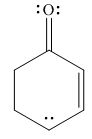
Figure 5
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
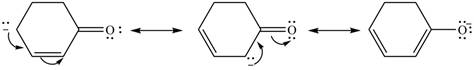
Figure 6
The possible resonance structure for the given species is shown in Figure 6.
(d)
Interpretation: The possible resonance structure for the given species is to be drawn.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.32P
The possible resonance structure for the given species is,
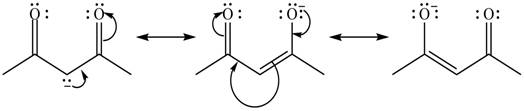
Explanation of Solution
The method by which overall delocalization of electrons can be described in a particular molecule is known as resonance.
The given species is shown below.
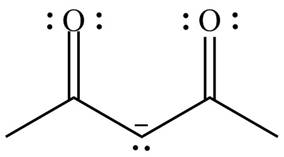
Figure 7
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.

Figure 8
The possible resonance structure for the given species is shown in Figure 8.
(e)
Interpretation: The possible resonance structure for the given species is to be drawn.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.32P
The possible resonance structure for the given species is,
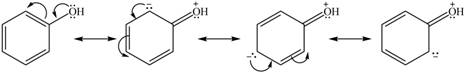
Explanation of Solution
The method by which overall delocalization of electrons can be described in a particular molecule is known as resonance.
The given species is shown below.
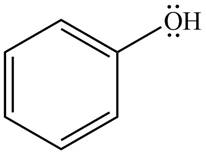
Figure 9
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
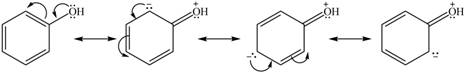
Figure 10
The possible resonance structure for the given species is shown in Figure 10.
(f)
Interpretation: The possible resonance structure for the given species is to be drawn.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.32P
The possible resonance structure for the given species is,
Explanation of Solution
The method by which overall delocalization of electrons can be described in a particular molecule is known as resonance.
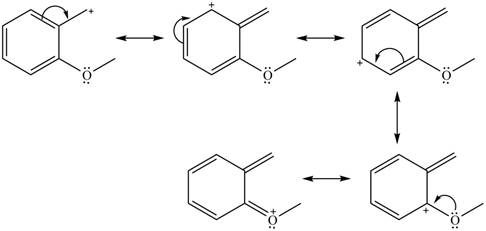
The given species is shown below.
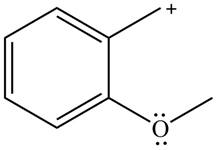
Figure 11
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.

Figure 12
The possible resonance structure for the given species is shown in Figure 12.
Want to see more full solutions like this?
Chapter 16 Solutions
PKG ORGANIC CHEMISTRY
- Problem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forwardSteps and explanationn please.arrow_forwardSteps and explanationn please.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





