
Concept explainers
(a)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the
Answer to Problem 16.32P
The name of the compound is 3,3-dimethylbutanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of four carbon atoms and two methyl substituentsare attached to carbon number 3.
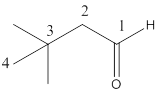
Therefore, the name of the compound becomes 3,3-dimethylbutanal.
(b)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon number 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
The name of the compound is 4-ethylheaxanal.
Explanation of Solution
The given molecular formula of the aldehyde is:
Here the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing aldehydic group consists of six carbon atoms and an ethyl substituent is attached to carbon number4.
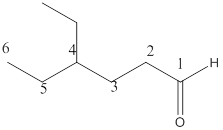
Therefore, the name of the compound becomes 4-ethylhexanal.
(c)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
The name of the compound is 3,4-dimethyloctanal.
Explanation of Solution
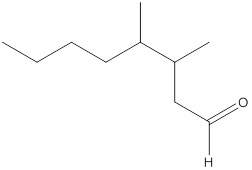
The given molecular formula of the aldehyde is:
Here, the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of eight carbon atoms and a methyl substituent is attached to carbon number 3 as well as carbon number 4.
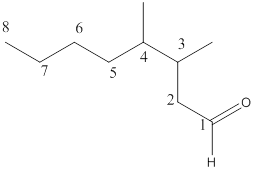
Therefore, the name of the compound becomes 3,4-dimethyloctanal.
(d)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon number 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
The name of the compound is 3-butylheptanal.
Explanation of Solution
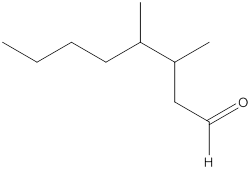
The given molecular formula of the aldehyde can be written as follows:
Here, the numbering of the carbon chain will start from the right where -CHO group is present. It can be clearly seen that the longest carbon chain containing the aldehyde group consists of seven carbon atoms and abutyl substituent is attached to carbon number 3.
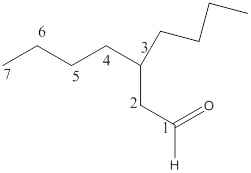
Therefore, the name of the compound becomes 3-butylheptanal.
(e)
Interpretation:
The name of the following aldehyde is to be determined.
Concept Introduction:
While naming the aldehydes as per the IUPAC nomenclature, the naming of the compounds is done by adding a suffix-al in the end of the name. Firstly, one will find the longest chain that contains the -CHO group and then change the -e ending of the parent alkane chain to -al suffix. Then, the numbering of the chain or the ring is done in such a way so as to put the -CHO group at carbon no. 1 followed by omitting this number from the name. Thereafter, apply all other rules of nomenclature as usual.
Answer to Problem 16.32P
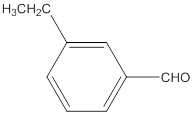
The name of the compound is 3-ethylbenzaldehyde.
Explanation of Solution
The given molecular formula of the aldehyde is:
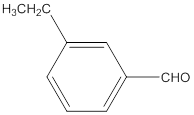
The name of the compound will be 3-ethylbenzaldehyde as the ethyl group is attached to the carbon number 3 of the parent molecule which is benzaldehyde. The benzene ring when attached to -CHO group is known as benzaldehyde.
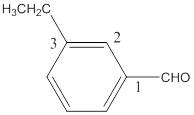
The naming of the molecule will start from the carbon to which aldehydic group is attached.
Want to see more full solutions like this?
Chapter 16 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- (a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forwardPlease helparrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning 
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





