
Concept explainers
a Draw a pH titration curve that represents the titration of 25.0 mL of 0.15 M propionic acid. CH3CH2COOH, by the addition of 0.15 M KOH from a buret. Label the axes and put a scale on each axis. Show where the equivalence point and the buffer region are on the titration curve. You should do calculations for the 0%, 50%, 60%, and 100% titration points. b Is the solution neutral, acidic, or basic at the equivalence point? Why?
(a)
Interpretation:
For titration of 25.0 mL of 0.15 M propionic acid,
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 50%, 60% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.120QP
A pH titration curve showing the equivalence point and buffer region is given in Figure 1 as follows,
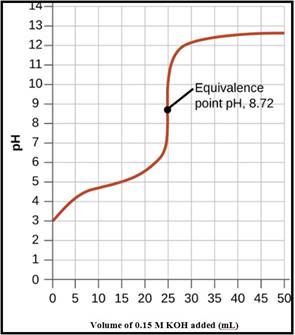
Figure 1
(a)
The pH at the 0% titration point is 2.85
The pH at the 50% titration point is 4.89
The pH at the 60% titration point is 5.06
The pH at the 100% titration point is 8.88
Explanation of Solution
To Draw: A pH titration curve showing the equivalence point and buffer region
The pH titration curve for the titration of 0.15 M propionic acid with 0.15 M
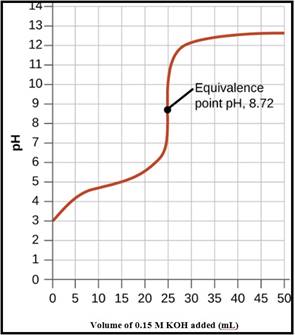
Figure 1
(a)
To Calculate: The pH of the titration points for the 0%, 50 %, 60% and 100%
Given data:
Titration of 0.15 M propionic acid with 0.15 M
pH at the 0% titration point:
Construct an equilibrium table with x as unknown concentration
Consider propionic acid as
|
|
|||
| Initial |
0.15
0.15-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Substitute equilibrium concentrations into the equilibrium-constant equation.
The
Assume x is negligible compared to 0.15 M
Therefore, the concentration of hydronium ion
In the end, pH is calculated as follows,
Therefore, the pH at the 0% titration point is 2.85
pH at the 50% titration point:
The pH is calculated as follows,
Therefore, the pH at the 50% titration point is 4.89
pH at the 60% titration point:
For convenience, express the concentrations as percents.
Substitute the concentrations into the equilibrium expression.
Therefore, the concentration of hydronium ion
In the end, pH is calculated as follows,
Therefore, the pH at the 60% titration point is 5.06
pH at the 100% titration point:
The salt that got produced has undergone a twofold dilution.
Therefore,
Construct an equilibrium table with x as unknown concentration
|
|
|||
| Initial |
0.0750
0.0750-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Now, calculate
Substitute into the equilibrium constant expression.
Here, x gives the concentration of hydroxide ion,
The pH is calculated as follows,
Therefore, the pH at the 100% titration point is 8.88
The pH at the 0% titration point was calculated as 2.85
The pH at the 50% titration point was calculated as 4.89
The pH at the 60% titration point was calculated as 5.06
The pH at the 100% titration point was calculated as 8.88
(b)
Interpretation:
For titration of 25.0 mL of 0.15 M propionic acid,
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 50%, 60% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.120QP
The solution at the equivalence point is basic
Explanation of Solution
To Explain: Whether the solution at the equivalence point is neutral, acidic or basic
As a result of titration Potassium propionate salt is produced.
Potassium propionate is the salt of a weak acid and a strong base.
Propionate ion reacts with water to produce hydroxide ions.
Therefore, the given solution is basic
The solution at the equivalence point was found as basic
Want to see more full solutions like this?
Chapter 16 Solutions
General Chemistry - Standalone book (MindTap Course List)
- Please answer the questions and provide detailed explanation. Please also include the Hydrogens that are on the molecule to show how many signals there are.arrow_forwardCapp aktiv.com Part of Speech Table for Assi x Aktiv Learning App K Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 232 of 10 10: Mg Select to Add Arrows Br O H :0 CI:O H Mg THE + dy Undo Reset Done Brarrow_forwardPlease answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked. Will the following reaction make a molecule with a new C – C bond as its major product: Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.arrow_forward
- Please answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked. Will the following reaction make a molecule with a new C – C bond as its major product: Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.arrow_forwardPlease do not use AI. AI cannot "see" the molecules properly, and it therefore gives the wrong answer while giving incorrect descriptions of the visual images we're looking at. All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forwardPlease answer the question and provide detailed explanations.arrow_forward
- All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forward5. Fill in the missing molecules in the following reaction pathway. TMSO Heat + CI then HF O₂N (1.0 equiv) AICI 3 OMearrow_forwarde. O₂N NO2 1. excess H2, Pd/C 2. excess NaNO2, HCI 3. excess CuCNarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





