
(a)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,
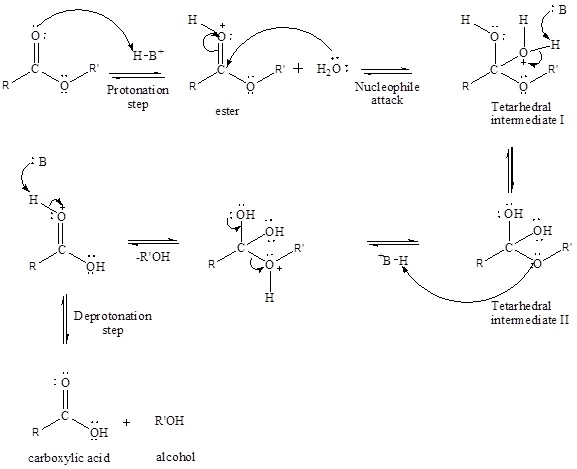
Therefore, products obtained by the acid catalyzed ester hydrolysis are the
(b)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction: An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
(c)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Student's Study Guide and Solutions Manual for Organic Chemistry
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning




