
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134649771
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 15, Problem 76P
Interpretation Introduction
Interpretation:
To give the explanation for aminolysis reaction of propionyl chloride and methylamine when one and two equivalents of methylamine is used.
Concept introduction:
Amine and acid chloride reacts to produce amide. In this reaction ofAcid chlorides and amine, a proton is also produced which protonates the unreacted
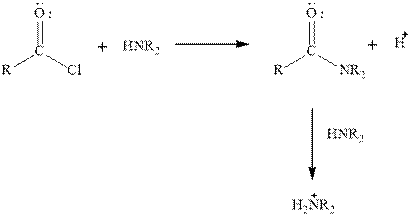
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the following acid-base reactions and predict the direction of equilibrium
for each. Justify your prediction by citing pK values for the acid and conjugate acid in
each equilibrium.
(a)
(b) NHs
(c)
O₂N
NH
NH
OH
H₁PO₁
23.34 Show how to convert each starting material into isobutylamine in good yield.
ཅ ནད ཀྱི
(b)
Br
OEt
(c)
(d)
(e)
(f)
H
Please help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.
Chapter 15 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
Ch. 15.1 - The aromas of many flowers and fruits are due to...Ch. 15.1 - Name the following:Ch. 15.1 - Prob. 3PCh. 15.2 - Which is longer, the carbon-oxygen single bond in...Ch. 15.2 - There are three carbon-oxygen bonds in methyl...Ch. 15.2 - Prob. 6PCh. 15.4 - a. What is the product of the reaction of acetyl...Ch. 15.4 - What is the product of an acyl substitution...Ch. 15.5 - a. Which compound has the stretching vibration for...Ch. 15.5 - Using the pKa values listed in Table 15.1, predict...
Ch. 15.5 - Is the following statement true or false? If the...Ch. 15.6 - Starting with acetyl chloride, what neutral...Ch. 15.6 - Prob. 13PCh. 15.7 - Starting with methyl acetate, what neutral...Ch. 15.7 - We saw that it is necessary to use excess amine in...Ch. 15.7 - Prob. 17PCh. 15.7 - Which ester hydrolyzes more rapidly? a. methyl...Ch. 15.7 - a. state three factors that cause the uncatalyzed...Ch. 15.8 - Prob. 21PCh. 15.8 - Using the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 23PCh. 15.8 - Show the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 25PCh. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.9 - Prob. 28PCh. 15.9 - Prob. 29PCh. 15.10 - Show how each of the following esters could he...Ch. 15.10 - Prob. 32PCh. 15.11 - Prob. 33PCh. 15.11 - Which of the following reactions leads to the...Ch. 15.12 - Prob. 35PCh. 15.12 - Prob. 36PCh. 15.13 - Prob. 37PCh. 15.14 - Prob. 38PCh. 15.14 - Prob. 39PCh. 15.15 - Prob. 40PCh. 15.15 - Which alkyl halides from the carboxylic acids...Ch. 15.16 - Prob. 43PCh. 15.16 - Prob. 44PCh. 15.16 - Prob. 45PCh. 15.17 - Prob. 46PCh. 15.18 - How could you synthesize the following compounds...Ch. 15 - Prob. 48PCh. 15 - Name the following:Ch. 15 - Prob. 50PCh. 15 - What compound are obtained from the fallowing...Ch. 15 - a. Rank the following esters in order of...Ch. 15 - Because bromocyclohexane is a secondary alkyl...Ch. 15 - a. Which compound would you expect to have a...Ch. 15 - How could you use 1H NMR spectroscopy to...Ch. 15 - Rank the following compounds in order of...Ch. 15 - Prob. 57PCh. 15 - Prob. 58PCh. 15 - Prob. 59PCh. 15 - A compound with molecular formula C5H10O2 gives...Ch. 15 - Prob. 61PCh. 15 - Prob. 62PCh. 15 - Prob. 63PCh. 15 - Prob. 64PCh. 15 - Prob. 65PCh. 15 - Prob. 66PCh. 15 - Two products, A and B, are obtained from the...Ch. 15 - Prob. 68PCh. 15 - Prob. 69PCh. 15 - Prob. 70PCh. 15 - Prob. 71PCh. 15 - Prob. 72PCh. 15 - When treated with an equivalent of methanol,...Ch. 15 - a. Identify the two products obtained from the...Ch. 15 - Prob. 75PCh. 15 - Prob. 76PCh. 15 - a. When a carboxylic acid is dissolved in...Ch. 15 - Prob. 78PCh. 15 - Identity the major and minor products of the...Ch. 15 - When a compound with molecular formula C11H14O2...Ch. 15 - Prob. 81PCh. 15 - Prob. 82PCh. 15 - Prob. 83PCh. 15 - The 1H NMR spectra for two esters with molecular...Ch. 15 - Show how the following compounds could be prepared...Ch. 15 - Prob. 86PCh. 15 - Prob. 87PCh. 15 - The intermediate shown here is formed during the...Ch. 15 - Prob. 89PCh. 15 - Propose a mechanism that accounts for the...Ch. 15 - Catalytic antibodies catalyze a reaction by...Ch. 15 - Prob. 92P
Knowledge Booster
Similar questions
- Propose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forward
- b. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forward
- Identify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole