
(a)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with
Concept introduction:
Esters can be prepared from the reaction of an alcohol with
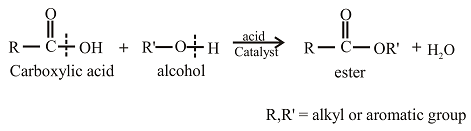
The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
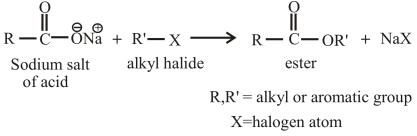
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,
(b)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(c)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(d)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
- What is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forwardIndicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forwardWhat are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



