
Interpretation:
To state the drawbacks of the reaction of
Concept introduction:
The synthesis of primary amine can be done by using three different methods.
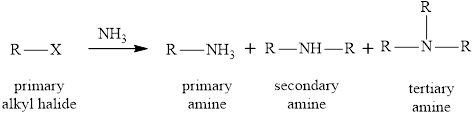
(1) In first method reaction of primary alkyl halide with ammonia is used as to prepare primary alkyl halide. This reaction is a nucleophilic substitution reaction in which ammonia molecule acts as a nucleophile and alkyl halide as the substrate. The products obtained after the reaction are mixture of primary, secondary and tertiary amines. Therefore, this method is not useful for the preparation of primary amines. The reaction equation is written as,
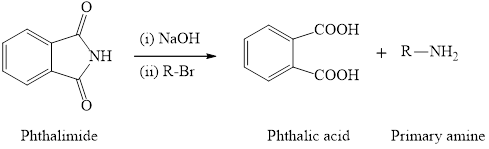
(2) The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
(3) The reaction of primary alkyl halide with azide ion forms alkyl azide which after catalytic hydrogenation reaction gives primary amine. This is the best method for the preparation of primary amines as the side product obtained is nitrogen gas which can be seperated from the primary amine easily. The reaction equation is written as,
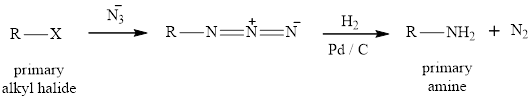
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forward
- Which of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forwardDraw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



