
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
6th Edition
ISBN: 9781266060144
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 44P
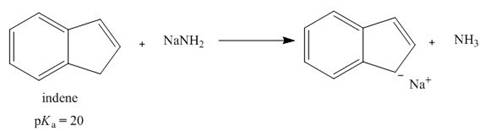
Treatment of indene with
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The number of noncyclic isomers that have the composition C4H8Owith the O as part of an OH group, counting a pair of stereoisomers as1, is A. 8; B. 6; C. 9; D. 5; E. None of the other answers is correct.
None
The number of carbon skeletons that have 8 carbons, one of which istertiary is A. 7; B. More than 7; C. 6; D. 5; E. 4
Chapter 15 Solutions
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
Ch. 15.2 - Prob. 1PCh. 15.2 - Problem 17.2 What orbitals are used to form the...Ch. 15.3 - Prob. 4PCh. 15.3 - Problem-17.5 What is the structure of propofol,...Ch. 15.6 - Prob. 7PCh. 15.8 - Prob. 8PCh. 15.8 - Prob. 11PCh. 15.8 - Prob. 12PCh. 15.8 - Problem 17.16 Rank the following compounds in...Ch. 15.8 - Problem 17.17 Draw the seven resonance structures...
Ch. 15 - 17.23 Name each compound and state how many lines...Ch. 15 - Prob. 21PCh. 15 - Prob. 22PCh. 15 - 17.27 Give the IUPAC name for each compounds.
a....Ch. 15 - 17.28 Draw a structure corresponding to each...Ch. 15 - 17.29 a. Draw the 14 constitutional isomers of...Ch. 15 - Prob. 26PCh. 15 - Prob. 27PCh. 15 - 17.38
How many electrons does C contain?
How...Ch. 15 - Prob. 36PCh. 15 - 17.40 Explain the observed rate of reactivity of...Ch. 15 - 17.41 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 39PCh. 15 - 17.43 Draw additional resonance structures for...Ch. 15 - Prob. 41PCh. 15 - Prob. 42PCh. 15 - 17.46 Which compound in each pair is the stronger...Ch. 15 - 17.47 Treatment of indene with forms its...Ch. 15 - Prob. 45PCh. 15 - 17.49 Draw the conjugate bases of pyrrole and...Ch. 15 - 17.50 a. Explain why protonation of pyrrole occurs...Ch. 15 - Prob. 48PCh. 15 - Prob. 49PCh. 15 - 17.53 How many signals does each compound...Ch. 15 - 17.54 Which of the diethylbenzene isomers (ortho,...Ch. 15 - 17.55 Propose a structure consistent with each...Ch. 15 - 17.56 Propose a structure consistent with each...Ch. 15 - 17.57 Thymol (molecular formula ) is the major...Ch. 15 - 17.58 You have a sample of a compound of molecular...Ch. 15 - 17.59 Explain why tetrahydrofuran has a higher...Ch. 15 - 17.60 Rizatriptan (trade name Maxalt) is a...Ch. 15 - 17.61 Zolpidem (trade name Ambien) promotes the...Ch. 15 - 17.62 Answer the following questions about...Ch. 15 - 17.63 Stanozolol is an anabolic steroid that...Ch. 15 - Prob. 61P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The azide ion is N3^-. In addition to the ionic charge, it’s three mostimportant contributing structures also have formal charges. The totalnumber of π bonds in these three contributing structures isA. 6; B. 12; C. 3; D. 9; E. None of the other answers is correct.arrow_forwardThe sum of the numerals in the name of the compoundis A. None of the other answers is correct.; B. 11;C. 6; D. 8; E. 5.arrow_forwardA compound has a six carbon ring with three double bonds. Attachedto the ring is a three carbon chain with a triple bond and a two carbonchain with two bromines attached. The number of hydrogens in a molecule of this compound is A. 10; B. 12; C. 14; D. 13; E. None of the other answers is correct.arrow_forward
- Can you help me? I can't seem to understand the handwriting for the five problems, and I want to be able to solve them and practice. If you'd like to give me steps, please do so to make it easier understand.arrow_forwardThe number of 2sp3 hybrid orbitals in the moleculeis A. 12; B. 8; C. 3; D. 11; E. None of the other answers is correct.arrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY