
(a)
Interpretation:
The statement “
(a)
Answer to Problem 3MCP
The given statement is false because amides shows a presence of strong electronegative oxygen atom of carboxyl group causes a very strong attraction between the lone pair of nitrogen electrons and the carbonyl group. Because, of which the unshared pair of electrons cannot hold a proton.
Explanation of Solution
Amide shows a presence of strong electronegative oxygen atom of carboxyl group causes a very strong attraction between the lone pair of nitrogen electrons and the carbonyl group. Because, of which the unshared pair of electrons cannot hold a proton.
Hence,
(b)
Interpretation:
The statement “
(b)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Amides have highest melting point when compared to alcohols and
The ability of primary and secondary amines to form
Hence,
(c)
Interpretation:
The statement “
(c)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(d)
Interpretation:
The statement “
(d)
Answer to Problem 3MCP
The given statement is false because
Explanation of Solution
The structure of
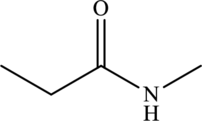
The carbonyl group present in
The statement “
(e)
Interpretation:
The statement “
(e)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
The structure of
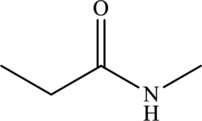
From the structure of
Hence, the given statement is true.
(f)
Interpretation:
The statement “
(f)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Amides have highest melting point when compared to
Hence, the given statement is true.
(g)
Interpretation:
The statement “
(g)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(h)
Interpretation:
The statement “
(h)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
(i)
Interpretation:
The statement “
(i)
Answer to Problem 3MCP
The given statement is false because it comprises of carbonyl functional group.
Explanation of Solution
Carboxyl group consists of carbonyl group and hydroxyl group.
The structure of
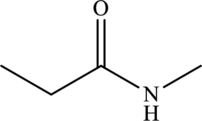
(j)
Interpretation:
The statement “
(j)
Answer to Problem 3MCP
The given statement is true.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of
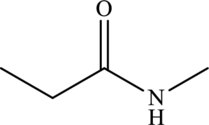
The molecular formula of
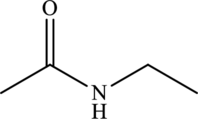
Both these are structural isomers with each other and hence, the statement “
Want to see more full solutions like this?
Chapter 15 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
- I have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forwardIf a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forward
- I have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forwardno Ai walkthroughsarrow_forwardno Ai walkthroughsarrow_forward
- no Ai walkthroughs (product in picture is wrong btw don't submit the same thing)arrow_forwardno Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





