
Concept explainers
Suggest appropriate methods for preparing each of the following
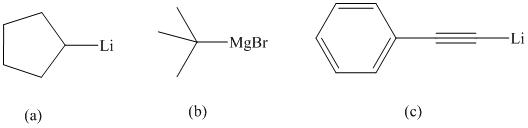
Interpretation:
The appropriate method of preparation for each of the given organometallic compounds is to be suggested.
Concept introduction:
The reaction of a metal with an organic halide is an oxidation–reduction in which the metal is the reducing agent.
lithium metal and magnesium metal in the presence of dry ether as a solvent, reacts with alkyl halides to produce the corresponding organo lithium and magnesium compounds respectively.
Organolithium reagents are used to prepare organometallic compounds analogous of terminal alkynes.
Organolithium compounds are strongly basic and react with terminal alkynes by abstracting the acidic proton and forming an alkane.
Answer to Problem 20P
Solution:

Explanation of Solution
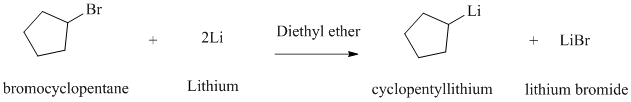
Alkyl halides react with lithium metal in the presence of dry ether as a solvent to produce the corresponding organo lithium compound.
The product given is cyclopentyl lithium. Reaction of cyclopentyl bromide with lithium metal in the presence of diethyl ether as a solvent will produce this cyclopentyl lithium.
The reaction is shown below:

The product given is tert-butyl magnesium bromide. Tert-butyl bromide react with magnesium metal in the presence of diethyl ether as a solvent will produce this tert-butyl magnesium bromide.
The reaction is shown below:

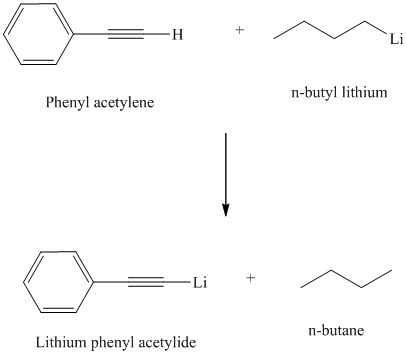
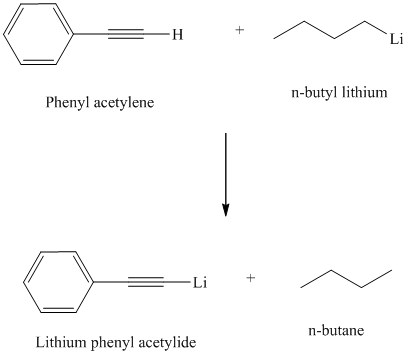
Organolithium reagents are used to prepare organometallic compounds analogous of terminal alkynes.
Organolithium compounds are strongly basic and react with terminal alkynes by abstracting the acidic proton and forming an alkane.
Thus, phenyl acetylene reacts with an organo lithium reagent like butyl lithium to produce lithium phenylacetylide and butane.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry - Standalone book
- Indicate the type of bond that is considered to be a hydrogen bond.(A). Permanent dipole-dipole interaction between polar molecules.(B). Mixed ionic-covalent bond.(C). Principal interatomic bond(D). Van del Waals forces.arrow_forwardRetro aldol: NaOH H₂O H NaOH & d H₂O Harrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. H conc. HBr Drawing Qarrow_forward
- Calculate the atomic packing factor of diamond knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are, respectively, 0.038 and 0.117 nm.arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows. Aldol: NaOH HO H Δ NaOH Δarrow_forwardNonearrow_forward
- Draw structures corresponding to the following names and give IUPAC names for the following compounds: (8 Point) a) b) c) CH3 CH2CH3 CH3CHCH2CH2CH CH3 C=C H3C H H2C=C=CHCH3 d) CI e) (3E,5Z)-2,6-Dimethyl-1,3,5,7-octatetraene f) (Z)-4-bromo-3-methyl-3-penten-1-yne g) cis-1-Bromo-2-ethylcyclopentane h) (5R)-4,4,5-trichloro-3,3-dimethyldecanearrow_forwardNonearrow_forwardReview: Design a total total synthesis synthesis of the following compound using methyloxacyclopropane and any other necessary reagents.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
