
Concept explainers
(a)
Interpretation:
To check whether the statement, “Aqueous solutions of
Concept Introduction:
Lewis acids are the ones which can accept a pair of electrons. Lewis bases are the ones which donate a pair of electrons. So, a substance which can donate a pair of electrons can be termed as a base.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

From the reaction, it is clear that the nitrogen donates a pair of electrons to hydrogen thus, making a salt. Thus, aqueous solutions of amines are basic in nature.
Hence, the statement is True.
(b)
Interpretation:
To check whether the statement, “
Concept Introduction:
Lewis acids are the ones which can accept a pair of electrons. Lewis bases are the ones which donate a pair of electrons. So, a substance which can donate a pair of electrons can be termed as a base.
Explanation of Solution
Aniline is the benzene ring that is attached to amine group. The lone pair of electrons present on the nitrogen atom is in conjugation with the aromatic ring. Thus, the lone pair of electrons is less available to donate to a proton. Hence, aniline is less basic in nature in comparison with methyl amine.
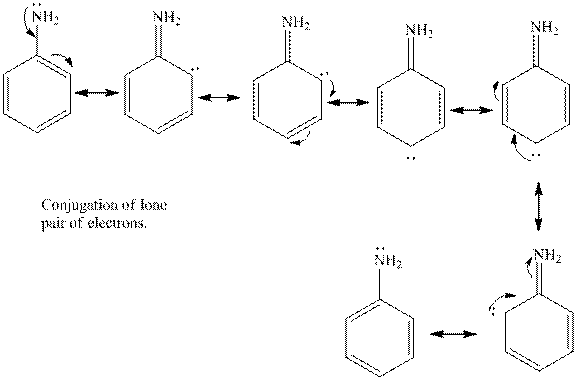
The cyclohexylamine is the one where the cyclohexyl ring is attached to the amine group. Hence, it is similar to an alkyl group attached to amine group. The alkyl groups are electron donating in nature due to inductive effect. Hence, the basicity of the amine nitrogen increases due to the attached cyclohexyl ring. Hence, the compound is more basic.
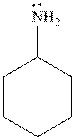
Thus, cyclohexylamine is more basic than aniline. Hence, aromatic amines are weaker bases than aliphatic amines.
The given statement is True.
(c)
Interpretation:
To check whether the statement, “Aliphatic amines are stronger bases than inorganic bases such as NaOH and KOH” is true or false.
Concept Introduction:
Generally, the strength of a base is compared on the basis that how readily the base produces the hydroxide ion. This can be either by releasing the hydroxide ion which they readily have and may be because they take hydrogen ions from the water and thus they can produce hydroxide ion.
Explanation of Solution
Generally, the strength of a base is compared on the basis that how readily the base produces the hydroxide ion. This can be either by releasing the hydroxide ion which they readily have and may be because they take hydrogen ions from the water and thus they can produce hydroxide ion.
NaOH and KOH are the acids which readily give hydroxide ions when added to water. They dissociate completely. The reaction is not reversible and hence, they are strong acids.
Aliphatic amines with general formula

Hence, the statement is False.
(d)
Interpretation:
To check whether the statement, “Water insoluble amines react with strong aqueous acids such as HCl to form water soluble salts” is true or false.
Concept Introduction:
Water has a property to ionize salts. This property helps in dissolution of salts in water.
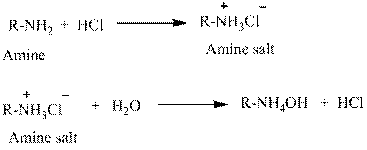
Explanation of Solution
Water is an universal solvent. Its polarity and many other aspects make it an unique solvent. It also has the property to ionize. This property makes salts to be easily dissolved in water. For this reason, amines which are usually insoluble in water are made soluble by converting into hydrochloride salts.
(e)
Interpretation:
To check whether the statement, “If the pH of an aqueous solution of a primary aliphatic amine is adjusted to pH 2.0 by the addition of concentrated HCl, the amine will be present in solution almost entirely as its conjugate acid” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. Given that pH is 2.0. That means, it is on the acidic side. As the acidic environment is present, the species that will present is the amine salt.
Hence, the statement is True.
(f)
Interpretation:
To check whether the statement, “If the pH of an aqueous solution of a primary aliphatic amine is adjusted to pH 10.0 by the addition of NaOH, the amine will be present in solution almost entirely as the free base” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. Given that pH is 10.0 by addition of NaOH. That means, the pH is on the basic side which is because of the added NaOH. As the basic environment is present, the species that will present is the basic amine.
Hence, the statement is True.
(g)
Interpretation:
To check whether the statement, “For a primary amine, the concentrations of salt and basic amine will be equal when the pH of the solution is equal to the pKb of the amine” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. At certain pH such as pKb the system is at equilibrium. pKb is the equilibrium dissociation constant pH. That means, the pH is so supporting that the forward and reverse reactions are at equal rate. That means, there will be forward and reverse reactions but that is not noticeable because they are at equal rate.
Hence, at this pH, the concentrations of salt and the basic salt will be equal. Hence, the statement is True.
Want to see more full solutions like this?
Chapter 15 Solutions
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
- 5.arrow_forward9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward
- 0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





