
(a)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
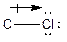
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
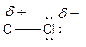
To find: Categorize all polar covalent bonds in the given compound (a)
(b)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
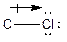
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
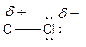
To find: Categorize all polar covalent bonds in the given compound (b)
(c)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
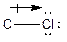
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
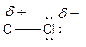
To find: Categorize all polar covalent bonds in the given compound (c)
(d)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
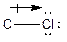
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
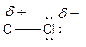
To find: Categorize all polar covalent bonds in the given compound (d)
(e)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
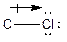
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
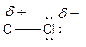
To find: Categorize all polar covalent bonds in the given compound (e)
(f)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
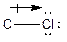
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
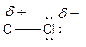
To find: Categorize all polar covalent bonds in the given compound (f)
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY 1 TERM ACCESS
- How will you prepare the following buffers? 2.5 L of 1.5M buffer, pH = 10.5 from NH4Cl and NH3arrow_forwardCH₂O and 22 NMR Solvent: CDCl3 IR Solvent: neat 4000 3000 2000 1500 1000 15 [ اند 6,5 9.8 3.0 7.0 6.0 5.0 4.8 3.0 2.0 1.0 9.8 200 100arrow_forwardprotons. Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x 1026arrow_forward
- Using what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





