
Interpretation:
The cell voltage at each volume of
Concept Introduction:-
Cell Voltage:
Cell voltage is the difference between cathode potential and anode potential.
The unreacted concentration in the solution is determined by
Ion-selective electrode:
An ion-selective electrode (ISE) best known as a specific ion electrode (SIE), is a sensor (or transducer) that transforms the activity of a specific ion dissolved in a solution into an electrical potential.
Ion-selective electrode obeys following equation.
Explanation of Solution
Given:
The titration reaction is a reduction reaction.
The cell voltage of the reaction is written as
Cell Voltage:
Cell voltage is the difference between cathode potential and anode potential.
Where,
Another titration reaction is
The solubility constant for
The given equivalence point is
The unreacted
At
At
At
At
After
At
At
At
All the obtained potential are plotted as,
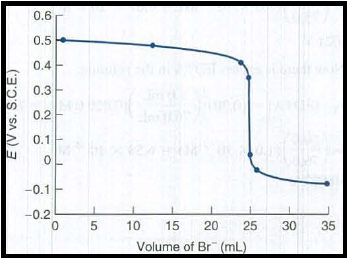
Figure 1
The cell voltage at each volume of
Want to see more full solutions like this?
Chapter 15 Solutions
Quantitative Chemical Analysis
- Name 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forward
- Under aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forward
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





