
Concept explainers
(a)
Interpretation:
From the given compounds, compounds with higher value of solubility has to be identified and the reason for the higher value of solubility has to be explained.
The given compounds are chloroethane and methylethylamine.
Concept Introduction:
The physical properties are determined by the intermolecular forces, polarity of the compound and hydrogen bonding. If a compound is polar and have hydrogen bonding, the solubility will be more for that compound than in non-polar compounds.
Intermolecular forces:
The forces which acts in between the atoms to hold a molecule are said to be intermolecular forces. They are weak forces. Intermolecular forces are categorized as hydrogen bonding, ionic bonding, ion-induced dipole forces, ion-dipole forces and Vanderwaal forces.
Hydrogen bonding:
The attraction between a lone pair of hydrogen atom with that of an electronegative atom forms hydrogen bonding. Two water molecules are held together by hydrogen bond.
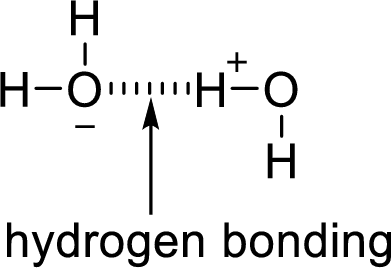
(b)
Interpretation:
From the given compounds, compounds with higher value of melting point has to be identified and the reason for the higher value of melting point has to be explained.
The given compounds are diethyl ether and 1-butanol.
Concept Introduction:
The physical properties are determined by the intermolecular forces, polarity of the compound and hydrogen bonding. If a compound is polar and have hydrogen bonding, the melting point will be more for that compound than in non-polar compounds.
Intermolecular forces:
The forces which acts in between the atoms to hold a molecule are said to be intermolecular forces. They are weak forces. Intermolecular forces are categorized as hydrogen bonding, ionic bonding, ion-induced dipole forces, ion-dipole forces and Vanderwaal forces.
Hydrogen bonding:
The attraction between a lone pair of hydrogen atom with that of an electronegative atom forms hydrogen bonding. Two water molecules are held together by hydrogen bond.
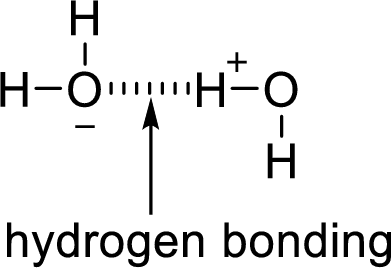
(c)
Interpretation:
From the given compounds, compounds with higher value of boiling point has to be identified and the reason for the higher value of boiling point has to be explained.
The given compounds are trimethylamine and propylamine.
Concept Introduction:
The physical properties are determined by the intermolecular forces, polarity of the compound and hydrogen bonding. If a compound is polar and have hydrogen bonding, the boiling point will be more for that compound than in non-polar compounds.
Intermolecular forces:
The forces which acts in between the atoms to hold a molecule are said to be intermolecular forces. They are weak forces. Intermolecular forces are categorized as hydrogen bonding, ionic bonding, ion-induced dipole forces, ion-dipole forces and Vanderwaal forces.
Hydrogen bonding:
The attraction between a lone pair of hydrogen atom with that of an electronegative atom forms hydrogen bonding. Two water molecules are held together by hydrogen bond.
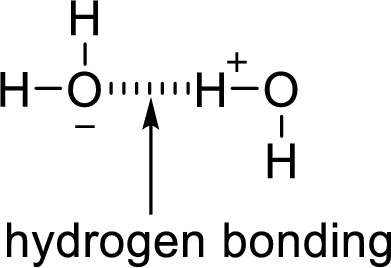
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
CHEMISTRY MOLECULAR NATURE OF MATTER AND
- Identify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forward
- H3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forward
- Is the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





