
(a)
Interpretation:
The effect of increased pressure over equilibrium for given system should be identified.
Concept Introduction:
Equilibrium: It is where the equal quantity of reactants and products exists as mixture, with the reaction in the forward and backward directions taking place simultaneously.
All
Le Chatelier's principle: When a change is applied to a system at equilibrium, the equilibrium will shift against the change. The equilibrium of a system will be affected by the change in temperature, pressure, and concentration.
Equilibrium Reaction:
Consider a simple equilibrium reaction as follows:
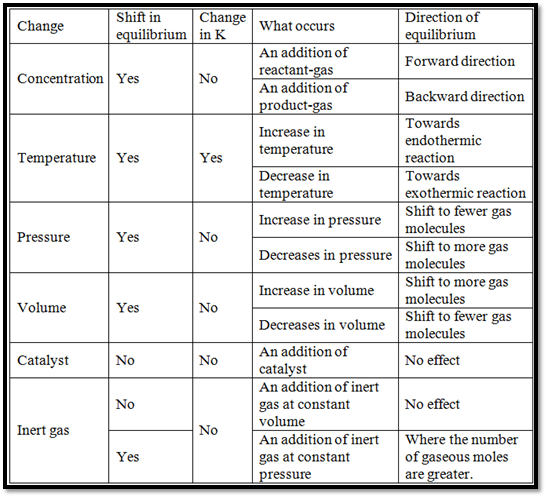
The Factors Affecting Equilibrium are given below:
(b)
Interpretation:
The effect of increased pressure over equilibrium for given system should be identified.
Concept Introduction:
Equilibrium: It is where the equal quantity of reactants and products exists as mixture, with the reaction in the forward and backward directions taking place simultaneously.
All chemical reactions strive to attain an equilibrium between the product side and the reactant side. The actual parameters defining the equilibrium could be the concentration of the respective species or pressure (in case of gaseous species).
Le Chatelier's principle: When a change is applied to a system at equilibrium, the equilibrium will shift against the change. The equilibrium of a system will be affected by the change in temperature, pressure, and concentration.
Equilibrium Reaction:
Consider a simple equilibrium reaction as follows:
The Factors Affecting Equilibrium are given below:

(c)
Interpretation:
The effect of increased pressure over equilibrium for given system should be identified.
Concept Introduction:
Equilibrium: It is where the equal quantity of reactants and products exists as mixture, with the reaction in the forward and backward directions taking place simultaneously.
All chemical reactions strive to attain an equilibrium between the product side and the reactant side. The actual parameters defining the equilibrium could be the concentration of the respective species or pressure (in case of gaseous species).
Le Chatelier's principle: When a change is applied to a system at equilibrium, the equilibrium will shift against the change. The equilibrium of a system will be affected by the change in temperature, pressure, and concentration.
Equilibrium Reaction:
Consider a simple equilibrium reaction as follows:
The Factors Affecting Equilibrium are given below:

(d)
Interpretation:
The effect of increased pressure over equilibrium for given system should be identified.
Concept Introduction:
Equilibrium: It is where the equal quantity of reactants and products exists as mixture, with the reaction in the forward and backward directions taking place simultaneously.
All chemical reactions strive to attain an equilibrium between the product side and the reactant side. The actual parameters defining the equilibrium could be the concentration of the respective species or pressure (in case of gaseous species).
Le Chatelier's principle: When a change is applied to a system at equilibrium, the equilibrium will shift against the change. The equilibrium of a system will be affected by the change in temperature, pressure, and concentration.
Equilibrium Reaction:
Consider a simple equilibrium reaction as follows:
The Factors Affecting Equilibrium are given below:

(e)
Interpretation:
The effect of increased pressure over equilibrium for given system should be identified.
Concept Introduction:
Equilibrium: It is where the equal quantity of reactants and products exists as mixture, with the reaction in the forward and backward directions taking place simultaneously.
All chemical reactions strive to attain an equilibrium between the product side and the reactant side. The actual parameters defining the equilibrium could be the concentration of the respective species or pressure (in case of gaseous species).
Le Chatelier's principle: When a change is applied to a system at equilibrium, the equilibrium will shift against the change. The equilibrium of a system will be affected by the change in temperature, pressure, and concentration.
Equilibrium Reaction:
Consider a simple equilibrium reaction as follows:
The Factors Affecting Equilibrium are given below:

Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
EBK GENERAL CHEMISTRY: THE ESSENTIAL CO
- I need the nomenclature of this compound.arrow_forwardI need the nomenclature of this compoundarrow_forward2. Name the following hydrocarbons. (9 marks) a) HHHHHHHH H-C-C- H-O-S b) HCEC-CH3 H H H H H d) c) H C=C- H H H e) CH3 CH3 CH2CH=CH-CH=CHCH3 HHHH H-C-C-C-C-H H HH H f) large CH2CH3 pola H3C section lovels tower, able ocart firs g) Tower H3C-CH2 then in H3C-CH-CH-CH3 enblbano bne noitsidab Copyright © 2008. Durham Continuing Education CH3arrow_forward
- Name the molecules & Identify any chiral center CH3CH2CH2CHCH₂CH₂CH₂CH₂ OH CH₂CHCH2CH3 Br CH3 CH3CHCH2CHCH2CH3 CH3arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





