
Concept explainers
Interpretation: The following question can be explained by the classification of carbohydrates.
Concept introduction: Carbohydrates are simple derivatives of polyhydroxy
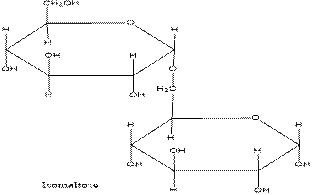
Given: Here we are given the Haworth structure of Isomaltose which we get from thebreakdown of starch and we are asked to find out the whether it is mono-, di-, or polysaccharide. We are also asked to find the monosaccharides and glycosidic link in isomaltose. Also, we need to find α or β-anomer of isomaltose, and whether it is a reducing sugar.
Answer to Problem 15.45UTC
Solution: (a) It is a disaccharide.
(b) The monosaccharides of Isomaltase are 2 molecules of α-glucose.
(c) The glycosidic link in Isomaltase is α-1,6-glycosidic bond.
(d) The given isomer is α-isomer.
Explanation of Solution
(a) In the given structure, there are 2 units of monosaccahrides. Thus, it is a disaccharide.
(b) If the molecule of Isomaltase is broken, then there is formation of 2 monosaccharide units of α-glucose molecules.
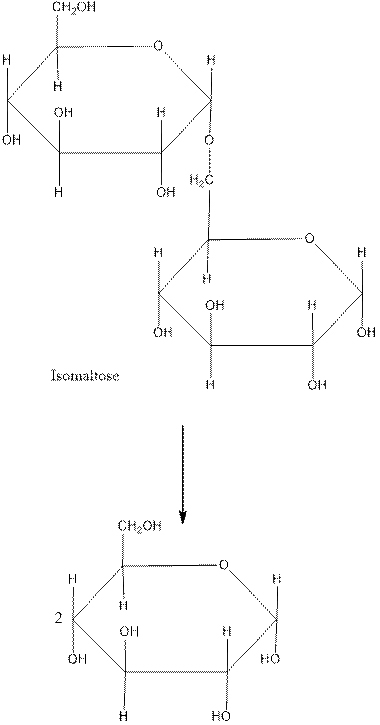
(c) The glycosidic bond in Isomaltase is formed between the C1 carbon of the 1st glucose molecule and the C6 carbon of the 2nd glucose. Thus the glycosidic link in Isomaltase is α-1, 6-glycosidic bond.
(d) When the hydroxyl group on C1 carbon atom is facing in downwards direction, then the isomer is called the α-isomer and when the hydroxyl group on C1 carbon atom is facing in upwards direction, then the isomer is called the β-isomer.
Here, the hydroxyl group on C1 carbon atom is facing in downwards direction, thus the given isomer is α-isomer.
Carbohydrates play important roles in the immune system, fertilization, blood clotting, overall development and preventing pathogenesis.
Want to see more full solutions like this?
Chapter 15 Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
- Highlight the chirality (or stereogenic) center(s) in the given compound. A compound may have one or more stereogenic centers. OH OH OH OH OH OHarrow_forwardUsing wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration. NH H Br X टेarrow_forwardProvide photos of models of the following molecules. (Include a key for identification of the atoms) 1,2-dichloropropane 2,3,3-trimethylhexane 2-bromo-3-methybutanearrow_forward
- Please draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





