
(a)
Interpretation:
The compound 1-pentene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(a)
Explanation of Solution
The given compound is 1-pentene.
The pentene contains five carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in 1-pentene is in between first and second carbon atoms. The structure of 1-pentene is given as
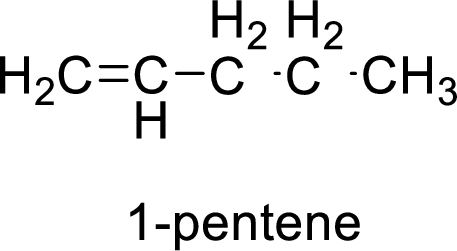
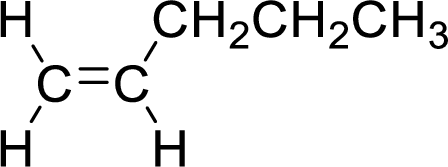
The given compound is 1-pentene. The given compound contains a double bond and each carbon in the double bond is bonded to two groups.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar groups are attached to the carbon on same side and in trans-isomer, similar groups are attached to the carbon on opposite sides.
As 1-pentene contains two similar groups (
(b)
Interpretation:
The compound 2-pentene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any functional groups are present in the compound, it also gives whether the compound is an alkane or alkene or alkyne.
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(b)
Explanation of Solution
The given compound is 2-pentene.
The pentene contains five carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in 2-pentene is in between second and third carbon atoms. The structure of 2-pentene is given as
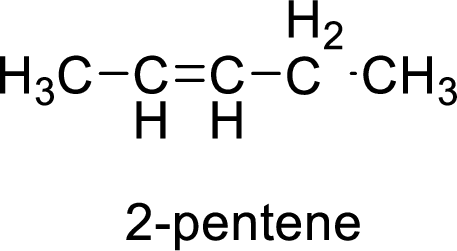
The given compound is 2-pentene. The given compound contains a double bond and each carbon in the double bond is bonded to two different groups. Hence, the given compound exhibits geometric isomerism.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar or higher priority groups are attached to the carbon on same side and in trans-isomer, similar or higher priority groups are attached to the carbon on opposite sides.
The groups present on either sides of the double bond are given as higher priority groups (
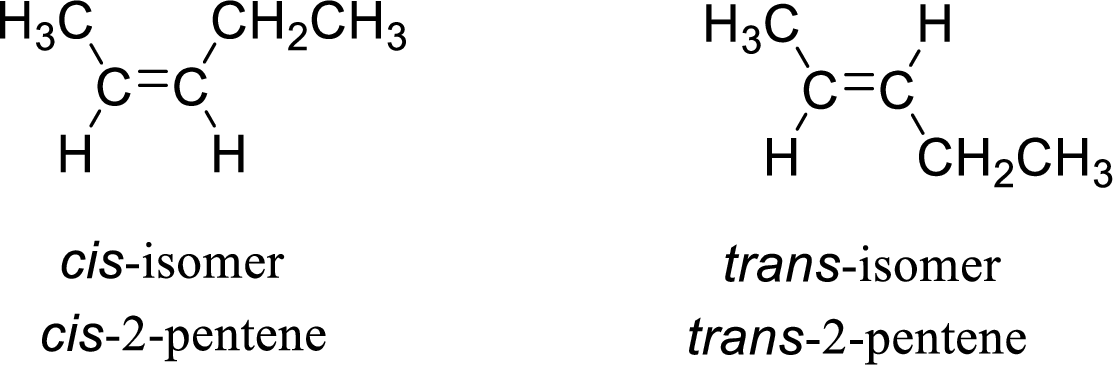
(c)
Interpretation:
The compound 1-chloropropene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any functional groups are present in the compound, it also gives whether the compound is an alkane or alkene or alkyne.
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(c)
Explanation of Solution
The given compound is 1-chloropropene.
The propene contains three carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in 1-chloropropene is in between first and second carbon atoms. One chloro group (
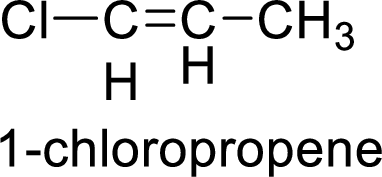
The given compound is 1-chloropropene. The given compound contains a double bond and each carbon in the double bond is bonded to two different groups. Hence, the given compound exhibits geometric isomerism.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar or higher priority groups are attached to the carbon on same side and in trans-isomer, similar or higher priority groups are attached to the carbon on opposite sides.
The groups present on either sides of the double bond are given as higher priority groups (
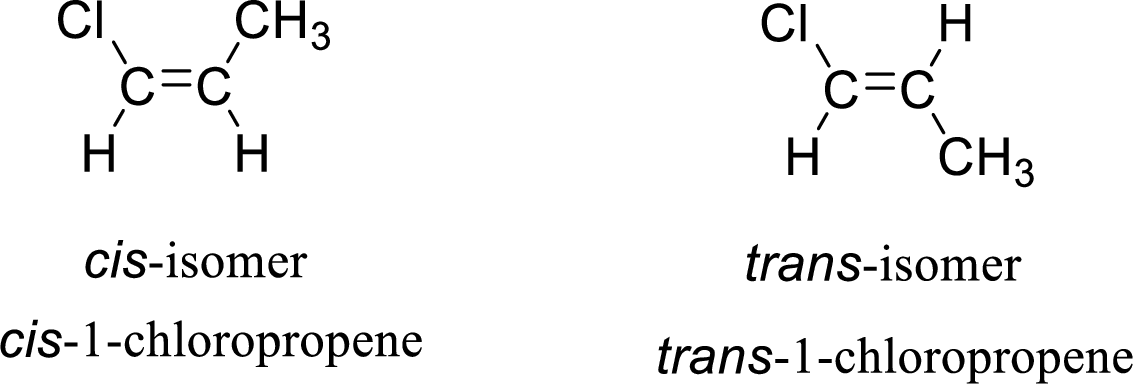
(d)
Interpretation:
The compound 2-chloropropene exhibit geometric isomerism or not has to be given and two isomers has to be drawn and named for the given structure.
Concept Introduction:
The structure of the compound is given by its systematic name.
To give the structure from the name of the compound, the root name has to be identified. The root name indicates the number of carbon atoms present in the longest chain.
Then the functional group (suffix) has to be identified. It indicates whether any functional groups are present in the compound, it also gives whether the compound is an alkane or alkene or alkyne.
The prefix of the name indicates the branched groups and their positions on the carbon chain.
The name of the compound is in the form
Prefix + Root + Suffix
The geometric isomers are said to be the isomers which shows different orientation of groups around a double bond. The geometric isomers are also known as cis-trans isomers.
When two similar or higher priority groups are attached to the carbon on same side, it is said to be cis-isomer.
When two similar or higher priority groups are attached to the carbon on opposite sides, it is said to be trans-isomer.
To exhibit the geometric isomerism, a molecule should have double bonded carbon atoms (
(d)
Explanation of Solution
The given compound is 2-chloropropene.
The propene contains three carbon atoms in chain. As the suffix is –ene, it belongs to alkene group and contains a double bond. The position of the double bond in 2-chloropropene is in between first and second carbon atoms. One chloro group (
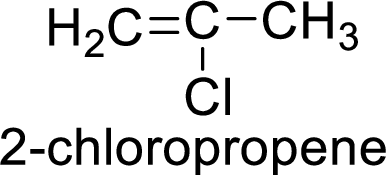
The given compound is 2-chloropropene. The given compound contains a double bond and each carbon in the double bond is bonded to two groups.
The geometric isomers are cis-isomer and trans-isomer. In cis-isomer, similar groups are attached to the carbon on same side and in trans-isomer, similar groups are attached to the carbon on opposite sides.
As 2-chloropropene contains two similar groups (
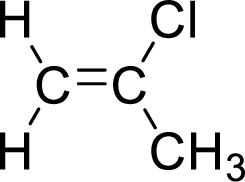
Want to see more full solutions like this?
Chapter 15 Solutions
Student Study Guide for Silberberg Chemistry: The Molecular Nature of Matter and Change
- (2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward(2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?arrow_forward8. (2 pts) Silicon semiconductors have a bandgap of 1.11 eV. What is the longest photon wavelength that can promote an electron from the valence band to the conduction band in a silicon-based photovoltaic solar cell? Show all work. E = hv = hc/λ h = 6.626 x 10-34 Js c = 3.00 x 108 m/s 1 eV 1.602 x 10-19 Jarrow_forward
- A solution containing 100.0 mL of 0.155 M EDTA buffered to pH 10.00 was titrated with 100.0 mL of 0.0152 M Hg(ClO4)2 in a cell: calomel electrode (saturated)//titration solution/Hg(l) Given the formation constant of Hg(EDTA)2-, logKf= 21.5, and alphaY4-=0.30, find out the cell voltage E. Hg2+(aq) + 2e- = Hg(l) E0= 0.852 V E' (calomel electrode, saturated KCl) = 0.241 Varrow_forwardFrom the following reduction potentials I2 (s) + 2e- = 2I- (aq) E0= 0.535 V I2 (aq) + 2e- = 2I- (aq) E0= 0.620 V I3- (aq) + 2e- = 3I- (aq) E0= 0.535 V a) Calculate the equilibrium constant for I2 (aq) + I- (aq) = I3- (aq). b) Calculate the equilibrium constant for I2 (s) + I- (aq) = I3- (aq). c) Calculate the solubility of I2 (s) in water.arrow_forward2. (3 pts) Consider the unit cell for the spinel compound, CrFe204. How many total particles are in the unit cell? Also, show how the number of particles and their positions are consistent with the CrFe204 stoichiometry - this may or may not be reflected by the particle colors in the diagram. (HINT: In the diagram, the blue particle is in an interior position while each red particle is either in a corner or face position.)arrow_forward
- From the following potentials, calculate the activity of Cl- in saturated KCl. E0 (calomel electrode)= 0.268 V E (calomel electrode, saturated KCl)= 0.241 Varrow_forwardCalculate the voltage of each of the following cells. a) Fe(s)/Fe2+ (1.55 x 10-2 M)//Cu2+ (6.55 x 10-3 M)/Cu(s) b) Pt, H2 (0.255 bar)/HCl (4.55 x 10-4 M), AgCl (sat'd)/Ag Fe2+ +2e- = Fe E0= -0.44 V Cu2+ + 2e- = Cu E0= 0.337 V Ag+ + e- = Ag E0= 0.799 V AgCl(s) + e- = Ag(s) + Cl- E0= 0.222 V 2H+ + 2e- = H2 E0= 0.000 Varrow_forwardA solution contains 0.097 M Ce3+, 1.55x10-3 M Ce4+, 1.55x10-3 M Mn2+, 0.097 M MnO4-, and 1.00 M HClO4 (F= 9.649 x 104 C/mol). a) Write a balanced net reaction that can occur between species in this solution. b) Calculate deltaG0 and K for the reaction. c) Calculate E and deltaG for the conditions given. Ce4+ + e- = Ce3+ E0= 1.70 V MnO4- + 8H+ + 5e- = Mn2+ + 4H2O E0= 1.507 Varrow_forward
- 1. Provide a step-by-step mechanism for formation of ALL STEREOISOMERS in the following reaction. Na HCO3 (Sodium bicarbonate, baking soda) is not soluble in CH2Cl2. The powder is a weak base used to neutralize strong acid (pKa < 0) produced by the reaction. Redraw the product to show the configuration(s) that form at C-2 and C-4. Br2 OH CH2Cl2 Na* HCO3 Br HO OH + Na Br +arrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O2/HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI + enant OH Solvent Reagent(s) Solvent Reagent(s)arrow_forwardGermanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap? Group of answer choices It is impossible to dope Ge and have this result in a larger bandgap. Dope the Ge with silicon (Si) Dope the Ge with gallium (Ga) Dope the Ge with phosphorus (P)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





