
Concept explainers
(a)
Interpretation: The most stable radical that can result from cleavage of
Concept introduction: A free radical is an atom or ion with unpaired electrons. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.2P
The most stable radical that can result from cleavage of
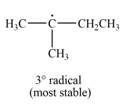
Explanation of Solution
The given species is,
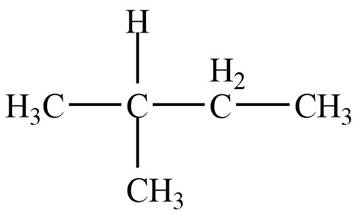
Figure 1
Three types of radicals can be formed by the cleavage of
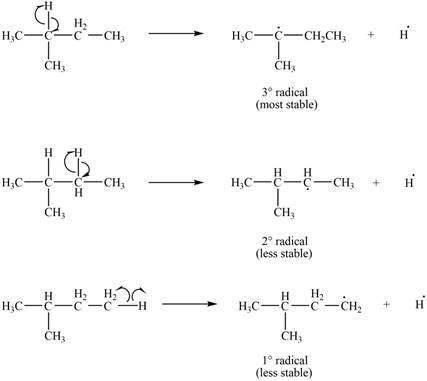
Figure 2
The number of alkyl substitutents increases, the stability of radical increases. The order of stability is
The most stable radical that can result from cleavage of
(b)
Interpretation: The most stable radical that can result from cleavage of
Concept introduction: A free radical is an atom or ion with unpaired electrons. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.2P
The most stable radical that can result from cleavage of
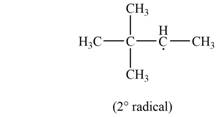
Explanation of Solution
The given species is,
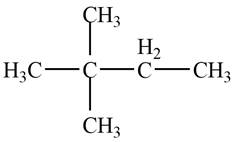
Figure 3
Two types of radicals can be formed from cleavage of
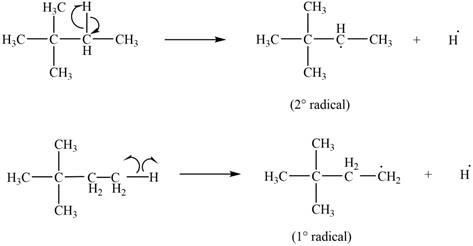
Figure 4
The number of alkyl substitutents increases, the stability of radical increases. The order of stability is
The most stable radical that can result from cleavage of
(c)
Interpretation: The most stable radical that can result from cleavage of
Concept introduction: A free radical is an atom or ion with unpaired electrons. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.2P
The most stable radical that can results from the cleavage of
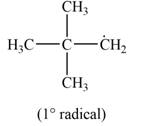
Explanation of Solution
The given species is,
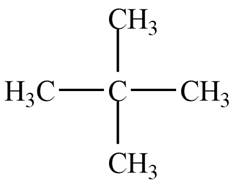
Figure 5
Only
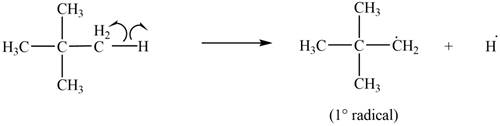
Figure 6
The most stable radical that can result from cleavage of
(d)
Interpretation: The most stable radical that can result from cleavage of
Concept introduction: A free radical is an atom or ion with unpaired electrons. They are reactive intermediates formed by the homolysis of covalent bond. Free radicals are classified as
Answer to Problem 15.2P
The most stable radical that can result from cleavage of
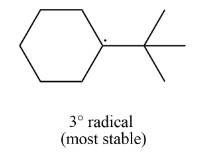
Explanation of Solution
The given species is,
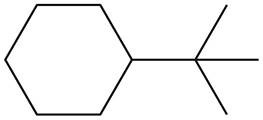
Figure 7
Two types of radicals can be formed from cleavage of
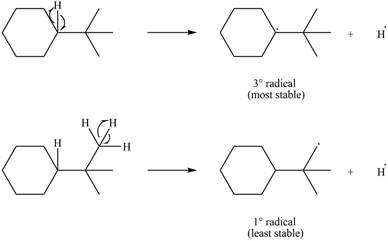
Figure 8
The number of alkyl substitutents increases, the stability of radical increases. The order of stability is
The most stable radical that can result from cleavage of
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY
- Predict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forwardDraw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forward
- Draw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forwardWhat is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forward
- What is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward> aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forward
- There are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forwardEEZE LETCHUP ID Draw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. Drawing く NaOCH2CH3 :0: :0: 狗arrow_forwardAnswerarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
