
Concept explainers
(a)
Interpretation:
Chalk should be classified as chiral or achiral.
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image. To consider as chiral, molecule or object and its mirror image should not superimpose. To consider as achiral, molecule or object and its mirror image should be superimposed with each other.
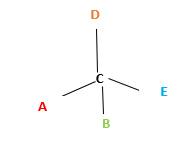
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different
Answer to Problem 15.25P
Chalk is achiral.
Explanation of Solution
To consider as chiral, molecule or object and its mirror image should not superimpose. To consider as achiral, molecule or object and its mirror image should be superimposed with each other. The mirror image of chalk is identical and is superimposable on their mirror images. Though they have not sharpened edges, the object and its mirror images are superimposable with each other. Thus, chalk is achiral.
(b)
Interpretation:
Pair of shoes should be classified as chiral or achiral.
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image. To consider as chiral, molecule or object and its mirror image should not superimpose. To consider as achiral, molecule or object and its mirror image should be superimposed with each other.
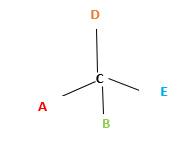
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different functional groups.
Answer to Problem 15.25P
Pair of shoes are chiral.
Explanation of Solution
To consider as chiral, molecule or object and its mirror image should not superimpose. To consider as achiral, molecule or object and its mirror image should be superimposed with each other. The mirror image of pair of shoes arenot identical and not superimposable. The object and its mirror images are not superimposable with each otherand not align all the parts of the objects with its mirror image. Thus, pair of shoes are chiral.
(c)
Interpretation:
A football should be classified as chiral or achiral.
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image. To consider as chiral, molecule or object and its mirror image should not superimpose. To consider as achiral, molecule or object and its mirror image should be superimposed with each other.
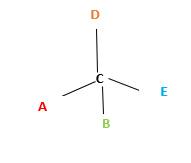
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different functional groups.
Answer to Problem 15.25P
Football is achiral.
Explanation of Solution
To be considered as chiral, molecule or object and its mirror image should not superimpose. To consider as achiral, molecule or object and its mirror image should be superimposed with each other. The mirror image of a football is identical and superimposable. The object and its mirror images are superimposable with each other. Thus, football is achiral.
Want to see more full solutions like this?
Chapter 15 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- Calculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forward
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
