
(a)
Interpretation:
MTBE (methyl tertiary-butyl ether) is synthesized by the catalyzed reaction of 2-methylpropene with methanol. The balanced equation for the synthesis of MTBE has to be written.
Concept Introduction:
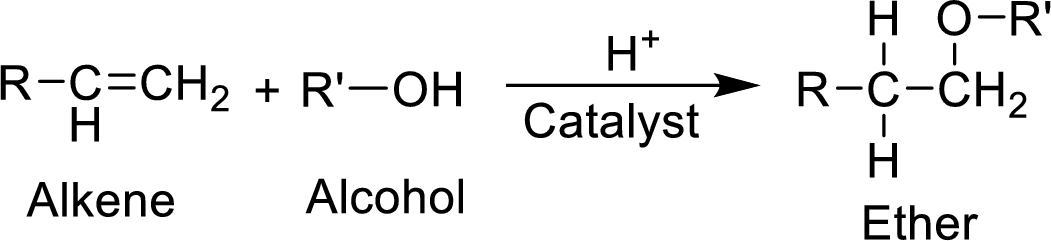
(a)
Explanation of Solution
MTBE is synthesized from 2-methylpropene and methanol. The reaction can be given as follows,
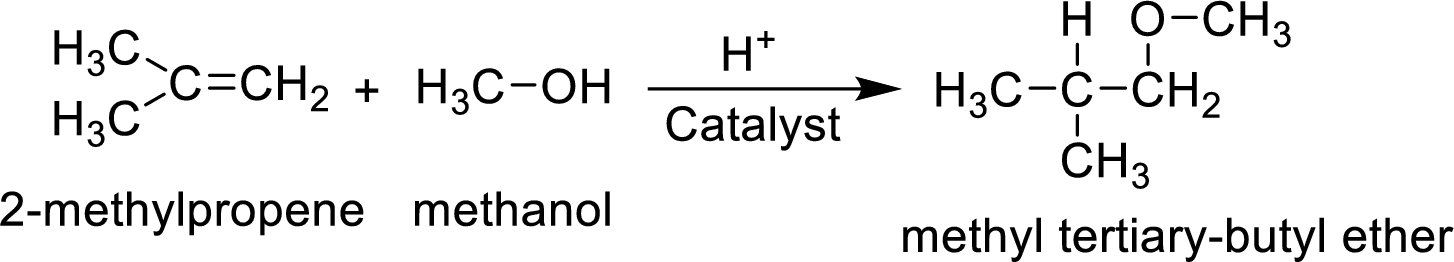
The reaction mechanism is as follows
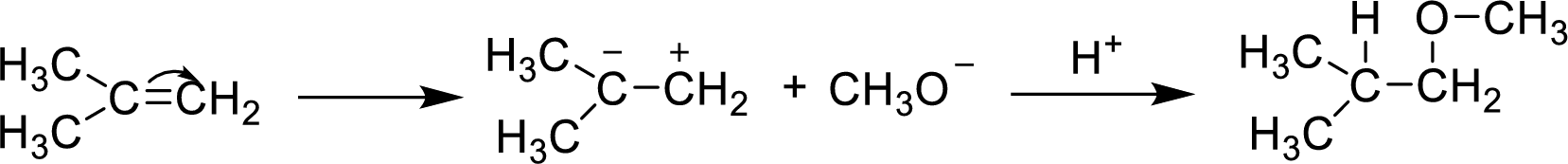
(b)
Interpretation:
If the government required auto fuel mixtures contain
Concept Introduction:
Mass percentage:
It is the concentration of an element or component in the total compound. It can be calculated as
Mass:
(b)
Explanation of Solution
Given,
The mass percentage of oxygen in MTBE has to be calculated.
The weight of oxygen is
Mass of MTBE can be calculated using the equation
The weight of the oxygen is given as
The mass of MTBE for
(c)
Interpretation:
The number of litres of MTBE would be in each litre of fuel mixture has to be calculated (density of both gasoline and MTBE is
Concept Introduction:
The volume of the compound can be calculated by using the density of that compound. Density can be calculated by using the equation
(c)
Explanation of Solution
The given density of gasoline and MTBE is
Mass of MTBE for
The volume of MTBE can be calculated as
The volume of MTBE in
(d)
Interpretation:
The number of litres of air (
Concept Introduction:
The ideal gas equation can be used to calculate the volume using pressure and temperature. The ideal gas equation is
Where,
(d)
Explanation of Solution
The balanced equation of MTBE on combustion with

The number of moles of oxygen required can be calculated as
Given,
The volume can be calculated as
The volume air required is
Want to see more full solutions like this?
Chapter 15 Solutions
CHEM 212:CHEMISTSRY V 2
- How will you prepare the following buffers? 2.5 L of 1.5M buffer, pH = 10.5 from NH4Cl and NH3arrow_forwardCH₂O and 22 NMR Solvent: CDCl3 IR Solvent: neat 4000 3000 2000 1500 1000 15 [ اند 6,5 9.8 3.0 7.0 6.0 5.0 4.8 3.0 2.0 1.0 9.8 200 100arrow_forwardprotons. Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x 1026arrow_forward
- Using what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





