
Concept explainers
a) 1 mol Br2 in CH2Cl2
Interpretation:
The products expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2 are to be given.
Concept introduction:
Conjugated dienes are treated with bromine in CH2Cl2 undergo both 1,2 and 1,4 addition of bromine to yield different products.
To give:
The product expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2.
Answer to Problem 27AP
The products expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2 are 3,6-dibromocyclohexene and 3,4-dibromocyclohexene.

Explanation of Solution
1,3-cyclohexadiene is a conjugated diene. When treated with one mole of bromine in CH2Cl2 it yields 3,6-dibromocyclohexene by 1,4 addition and 3,4-dibromocyclohexene by 1,2 addition reaction.
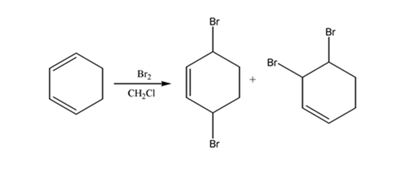
The products expected to be formed when 1,3-cyclohexadiene reacts with one mole of bromine in CH2Cl2 are 3,6-dibromocyclohexene and 3,4-dibromocyclohexene.

b) O3 followed by Zn
Interpretation:
The product expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc is to be given.
Concept introduction:
To give:
The product expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc is to be given.
Answer to Problem 27AP
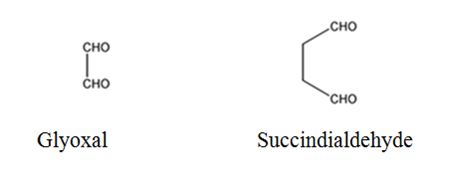
The products expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc are glyoxal and succindialdehyde.
Explanation of Solution
Ozone adds to both the double bonds in 1,3-cyclohexadiene to form an ozonide. When treated with Zn and acetic acid the ozonide breaks to yield carbonyl compounds. The carbons that have oxygen in the products are joined through double bonds in the reactant.
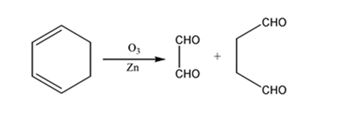
The products expected to be formed when 1,3-cyclohexadiene is treated with ozone followed by zinc are glyoxal and succindialdehyde.

c) 1 mol HCl in ether
Interpretation:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl in ether is to be given.
Concept introduction:
Conjugated dienes when treated with one mole of HCl in ether undergo both 1,2 and 1,4 addition of HCl to yield different products. With unsubstituted cyclic conjugated 1,3-dienes a single product is produced in both types of addition.
To give:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl is to be given.
Answer to Problem 27AP
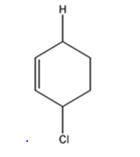
The product expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl is 3-chlorocyclohexene.
Explanation of Solution
Both 1,2 and 1,4 addition of HCl to 1,3-cyclohexadiene leads to the formation of the same product, 3-chlorocyclohexene, as it is an unsubstituted conjugated 1,3-diene.
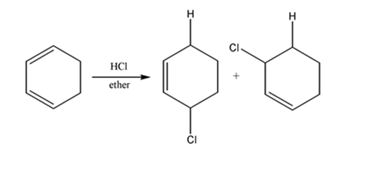
The product expected to be formed when 1,3-cyclohexadiene is treated with one mole of HCl is 3-chlorocyclohexene.
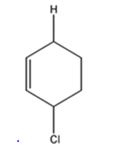
d) 1 mol DCl in ether
Interpretation:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl are to be given.
Concept introduction:
Conjugated dienes when treated with one mole of DCl in ether undergo both 1,2 and 1,4 addition of DCl to yield different products.
To give:
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl.
Answer to Problem 27AP
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl are 3-chloro-4-deuteratedcyclohexene and 3-chloro-6-deuteratedcyclohexene.
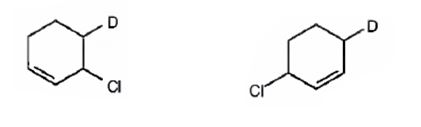
Explanation of Solution
1,3-cyclohexadiene is a conjugated diene. When treated with one mole of DCl it yields 3-chloro-4-deuteratedcyclohexen by 1,2 addition 3-chloro-6-deuteratedcyclohexene by 1,4 addition reaction.
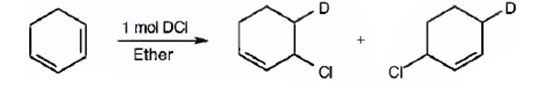
The products expected to be formed when 1,3-cyclohexadiene is treated with one mole of DCl are 3-chloro-4-deuteratedcyclohexene and 3-chloro-6-deuteratedcyclohexene.

e) 3-Buten-2-one (H2C=CHCOCH3)
Interpretation:
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one is to be given.
Concept introduction:
In Diels-Alder reaction, a dienophile reacts with a diene to yield a cyclic adduct. The reaction takes place through 1,4 addition of the dienophile into the diene through a cyclic transition state. During the reaction the diene and dienophile orient themselves on top of one another and so an endo product results.
To give:
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one.
Answer to Problem 27AP
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one is
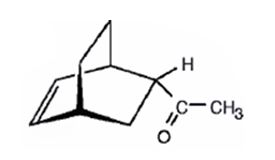
Explanation of Solution
The diene, 1,3-cyclohexadiene and the dienophile 3-buten-2-one arrange themselves one on the top of the other and react through the formation of a cyclic six member transition state to yield the endo product.
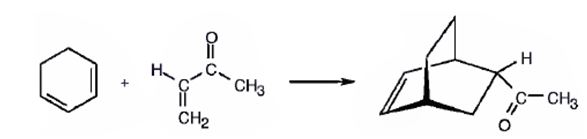
The product expected to be formed when 1,3-cyclohexadiene is treated with 3-buten-2-one is
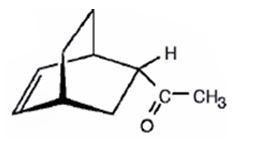
f) Excess OsO4, followed by NaHSO3
Interpretation:
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3 is to be given.
Concept introduction:
The reaction given involves hydroxylation of the double bonds in the diene. When a diene is treated with OsO4, a single step addition of OsO4 to each of the double bonds takes place to give a cyclic osmate. The cyclic osmate gets cleaved when treated with NaHSO3 yields a tetraol.
To give:
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3.
Answer to Problem 27AP
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3 is cyclohexane-1,2,3,4-tetraol.
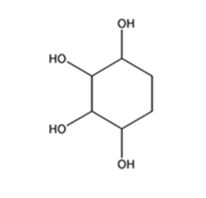
Explanation of Solution
When 1,3-cyclohexadiene is treated with excess OsO4 the addition of OsO4 takes place to both the double bonds in it. The addition occurs with syn stereochemistry to yield a cyclic osmate. The cyclic osmate then gets cleaved when treated with NaHSO3 to yield the tetraol.
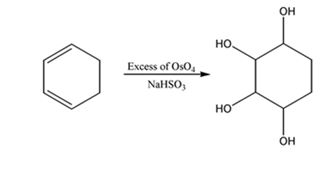
The product expected to be formed when 1,3-cyclohexadiene is treated with excess OsO4 and then with NaHSO3 is cyclohexane-1,2,3,4-tetraol.
Want to see more full solutions like this?
Chapter 14 Solutions
EBK ORGANIC CHEMISTRY
- The decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forwardDon't used hand raitingarrow_forwardCS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forward
- CS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forwardControl Chart Drawing Assignment The table below provides the number of alignment errors observed during the final inspection of a certain model of airplane. Calculate the central, upper, and lower control limits for the c-chart and draw the chart precisely on the graph sheet provided (based on 3-sigma limits). Your chart should include a line for each of the control limits (UCL, CL, and LCL) and the points for each observation. Number the x-axis 1 through 25 and evenly space the numbering for the y-axis. Connect the points by drawing a line as well. Label each line drawn. Airplane Number Number of alignment errors 201 7 202 6 203 6 204 7 205 4 206 7 207 8 208 12 209 9 210 9 211 8 212 5 213 5 214 9 215 8 216 15 217 6 218 4 219 13 220 7 221 8 222 15 223 6 224 6 225 10arrow_forward
- Collagen is used to date artifacts. It has a rate constant = 1.20 x 10-4 /years. What is the half life of collagen?arrow_forwardיווי 10 20 30 40 50 60 70 3.5 3 2.5 2 1.5 1 [ppm] 3.5 3 2.5 2 1.5 1 6 [ppm] 1 1.5 -2.5 3.5arrow_forward2H2S(g)+3O2(g)→2SO2(g)+2H2O(g) A 1.2mol sample of H2S(g) is combined with excess O2(g), and the reaction goes to completion. Question Which of the following predicts the theoretical yield of SO2(g) from the reaction? Responses 1.2 g Answer A: 1.2 grams A 41 g Answer B: 41 grams B 77 g Answer C: 77 grams C 154 g Answer D: 154 grams Darrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





