
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.7, Problem 14P
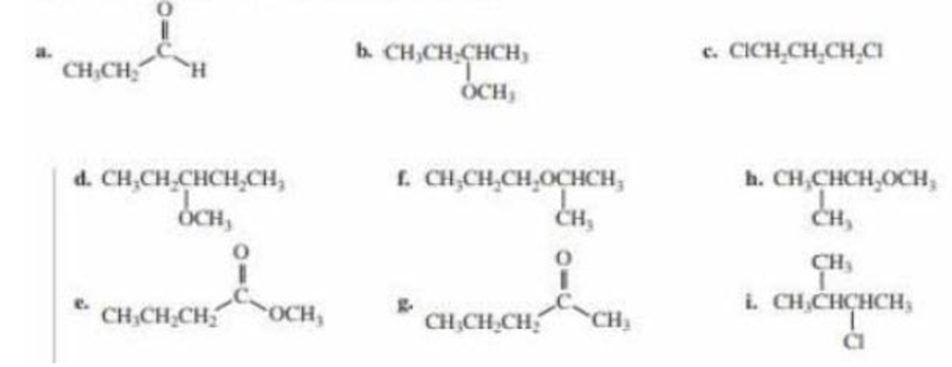
Without referring to Table 14.1, label the proton or set of protons in each compound that gives the signal at the lowest frequency a, at the next lowest b, and so on.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the following alkyne, complete the reaction sequentially (that is draw the intermediate that we can’t stop at) and then name (complete name) all 3 molecules.
Given the reaction sequence below, answer the following.
A. Provide the structure for A.
B. Provide the structure for B (pay attention to stereochemistry).
C. Provide the structure for C.
D. What are the stereochemical designations for I and II (R/S)?
Which of the following is the most stable carbon radical?
Chapter 14 Solutions
EBK ORGANIC CHEMISTRY
Ch. 14.1 - Prob. 1PCh. 14.1 - Prob. 2PCh. 14.4 - How many signals would you expect to see in the 1H...Ch. 14.4 - How many signals would you expect to see in the 1H...Ch. 14.4 - How could you distinguish the 1H NMR spectra of...Ch. 14.4 - Draw an isomer of dichlorocyclopropane that gives...Ch. 14.5 - Prob. 7PCh. 14.5 - Prob. 8PCh. 14.5 - Prob. 9PCh. 14.5 - Where would you expect to find the 1H NMR signal...
Ch. 14.6 - Prob. 11PCh. 14.7 - Prob. 12PCh. 14.7 - Prob. 13PCh. 14.7 - Without referring to Table 14.1, label the proton...Ch. 14.8 - [18]-Annulene shows two signals in its 1H NMR...Ch. 14.9 - How would integration distinguish the 1H NMR...Ch. 14.9 - Which of the following compounds is responsible...Ch. 14.10 - Prob. 19PCh. 14.10 - Explain how the following compounds, each with the...Ch. 14.10 - The 1H NMR spectra of two carboxylic acids with...Ch. 14.11 - Draw a diagram like the one shown in Figure 14.12...Ch. 14.12 - Indicate the number of signals and the...Ch. 14.12 - Explain the relative chemical shifts of the...Ch. 14.12 - How can their 1H NMR spectra distinguish the...Ch. 14.12 - Identify each compound from its molecular formula...Ch. 14.12 - Predict the splitting patterns for the signals...Ch. 14.12 - Describe the 1H NMR spectrum you would expect for...Ch. 14.12 - Propose structures that are consistent with the...Ch. 14.13 - Prob. 30PCh. 14.13 - Identify the compound with molecular formula...Ch. 14.14 - Prob. 32PCh. 14.15 - a. For the following compounds, which pairs of...Ch. 14.15 - How would the 1H NMR spectra for the four...Ch. 14.17 - Explain why the chemical shift of the OH proton of...Ch. 14.17 - Prob. 38PCh. 14.17 - Prob. 39PCh. 14.17 - Prob. 40PCh. 14.20 - Answer the following questions for each compound:...Ch. 14.20 - Prob. 42PCh. 14.20 - How can 1,2-, 1,3-, and 1,4-dinitrobenzene be...Ch. 14.20 - Identify each compound below from its molecular...Ch. 14.22 - Prob. 45PCh. 14.22 - What does cross peak X in Figure 14.34 tell you?Ch. 14 - Prob. 47PCh. 14 - Draw a spitting diagram for the Hb proton and give...Ch. 14 - Label each set of chemically equivalent protons,...Ch. 14 - Determine the ratios of the chemically...Ch. 14 - How can 1H NMR distinguish between the compounds...Ch. 14 - Prob. 52PCh. 14 - Match each of the 1H NMR spectra with one of the...Ch. 14 - The 1H NMR spectra of three isomers with molecular...Ch. 14 - Prob. 55PCh. 14 - Prob. 56PCh. 14 - Compound A, with molecular formula C4H9Cl, shows...Ch. 14 - Would it be better to use 1H NMR or 13C NMR...Ch. 14 - There are four esters with molecular formula...Ch. 14 - Identify the compound with molecular formula C6H14...Ch. 14 - An alkyl halide reacts with an alkoxide ion to...Ch. 14 - The 1H NMR spectra of three isomers with molecular...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Prob. 65PCh. 14 - How can the signals in the 6.5 to 8.1 ppm region...Ch. 14 - The 1H NMR spectra of two compounds, each with...Ch. 14 - Draw a splitting diagram for the Hb proton if Jbc...Ch. 14 - Sketch the following spectra that would be...Ch. 14 - How can 1H NMR be used to prove that the addition...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Dr. N. M. Arr was called in to help analyze the 1H...Ch. 14 - Calculate the amount of energy (in calories)...Ch. 14 - The following 1H NMR spectra are four compounds,...Ch. 14 - When compound A (C5H12O) is treated with HBr, it...Ch. 14 - Identity the compound with molecular formula...Ch. 14 - Identity each of the following compounds from its...Ch. 14 - Prob. 78PCh. 14 - Identify each of the following compounds from its...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Put the following carbon radicals in order of increasing stability.arrow_forwardDraw the major organic product for each of the following reactions (pay attention to stereochemistry).arrow_forwardThere are 2 reactions (that you know of) to achieve the following transformation: One reaction is favored over the other because it avoids a competing reaction. A. Draw the favored reaction scheme (not the mechanism), be sure to include all necessary reagents. B. Draw the reaction scheme that is not favored and include all the possible products.arrow_forward
- Both carbocations and carbon-radicals have trigonal planar geometry. True or Falsearrow_forwardTeflon (polytetrafluoroethene) is prepared via the radial polymerization of tetrafluoroethene. What other reaction conditions (reagent, etc.) are needed to accomplish this? A. NBS, Light B. Heat, Cl2 C. Peroxide, Heat D. H2SO4, H2O, Heatarrow_forwardWhich of the following compounds can be reacted with ethene to prepare 1,1- dichlorocyclopropane? A. CCl4 B. CCl2 C. CHCl3 D. CH2Cl2arrow_forward
- CI 4. How are the products of the following reaction related? (assuming we can control the chlorination as given by the reaction) C Cl2, light A. Enantiomers B. Constitutional isomers C. Regioisomers D. Diastereomers C +arrow_forwardVinyl and allyl radicals are equally stable due to resonance stabilization True OR Falsearrow_forwardAll of the following are true of Markovnikov’s rule EXCEPT A. The nucleophile adds to the most substituted carbon B. The more stable carbocation is formed in the transition state C. The electrophile adds to the carbon that has the most hydrogens D. There are no exceptions to this rulearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY