
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 14.2C, Problem 14.1P
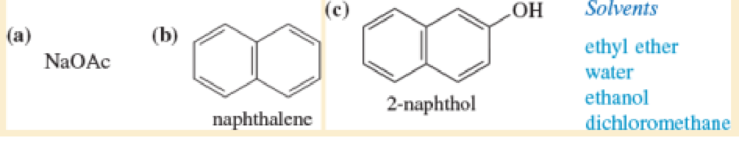
Rank the given solvents in decreasing order of their ability to dissolve each compound.
Solutes
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule03:52
Students have asked these similar questions
Draw the structure of the product of the reaction given the IR and MS data.
Spectral analysis of the product reveals:
MS: M 150, M-15, M-43
CH.COCI
AICI,
IR: 3150-3000 cm, 2950-2850 cm
and 1700 cm
Part II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic.
a)
HO
b)
Bri
H
HH
c)
d)
H
H H Br
0
None
Chapter 14 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 14.2C - Rank the given solvents in decreasing order of...Ch. 14.2D - Prob. 14.2PCh. 14.2D - In the presence of 18-crown-6, potassium...Ch. 14.3B - Give a common name (when possible) and a...Ch. 14.3C - Prob. 14.5PCh. 14.3C - Name the following heterocyclic ethers.Ch. 14.4 - Propose a fragmentation to account for each...Ch. 14.5 - Prob. 14.8PCh. 14.5 - Prob. 14.9PCh. 14.6 - Prob. 14.10P
Ch. 14.7 - Explain why bimolecular condensation is a poor...Ch. 14.7 - Prob. 14.12PCh. 14.7 - Prob. 14.13PCh. 14.8 - Prob. 14.14PCh. 14.8 - Prob. 14.15PCh. 14.8 - Prob. 14.16PCh. 14.10A - Prob. 14.17PCh. 14.10A - Prob. 14.18PCh. 14.10B - Prob. 14.19PCh. 14.11B - Show how you would accomplish the following...Ch. 14.11B - Prob. 14.21PCh. 14.12 - Prob. 14.22PCh. 14.12 - Prob. 14.23PCh. 14.12 - Prob. 14.24PCh. 14.13 - Prob. 14.25PCh. 14.13 - Prob. 14.26PCh. 14.14 - Prob. 14.27PCh. 14.15 - Give the expected products of the following...Ch. 14 - Write structural formulas for the following...Ch. 14 - Give common names for the following compounds. a....Ch. 14 - Give IUPAC names for the following compounds. a....Ch. 14 - Glycerol (propane-1,2,3-triol) is a viscous syrup...Ch. 14 - Prob. 14.33SPCh. 14 - Show how you would make the following ethers,...Ch. 14 - (A true story.) An inexperienced graduate student...Ch. 14 - Prob. 14.36SPCh. 14 - a. Show how you would synthesize the pure (R)...Ch. 14 - a. Predict the values of m/z and the structures of...Ch. 14 - The following reaction resembles the...Ch. 14 - Prob. 14.40SPCh. 14 - Prob. 14.41SPCh. 14 - Prob. 14.42SPCh. 14 - Give the structures of the intermediates...Ch. 14 - Prob. 14.44SPCh. 14 - Show how you would synthesize the following ethers...Ch. 14 - Prob. 14.46SPCh. 14 - Prob. 14.47SPCh. 14 - Prob. 14.48SPCh. 14 - An acid-catalyzed reaction was carried out using...Ch. 14 - Propylene oxide is a chiral molecule. Hydrolysis...Ch. 14 - Prob. 14.51SPCh. 14 - Prob. 14.52SPCh. 14 - Prob. 14.53SPCh. 14 - Prob. 14.54SPCh. 14 - In 2012, a group led by Professor Masayuki Satake...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
8.63 Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g ...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
What two body structures contain flexible elastic cartilage?
Anatomy & Physiology (6th Edition)
Modified True/False 6. __________ Halophiles inhabit extremely saline habitats, such as the Great Salt Lake.
Microbiology with Diseases by Body System (5th Edition)
Alkaptonuria is an infrequent autosomal recessive condi-tion. It is first noticed in newborns when the urine in...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY