
Concept explainers
Each of the following compounds is characterized by a 1H NMR spectrum that consists of
only a single peak having the chemical shift indicated. Identify each compound.
Interpretation:
The compounds gives only single peak in 1H NMR spectrum as indicated by the chemical shift is to be identified.
Concept introduction:
The information related to the proton in the compound can be deduced by the number of signals in a spectrum.
The proton chemical shift in the compound is due to the environment of the proton.
The signal of a particular proton can be split by the presence of protons in vicinal position.
The NMR spectrum of a compound will consist of a single peak if all protons in the compound are structurally equivalent.
Index of hydrogen deficiency is calculated as
Here
Oxygen atoms do not affect the index of hydrogen deficiency.
Answer to Problem 31P
Solution:
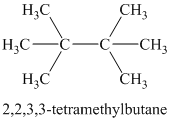
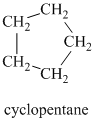
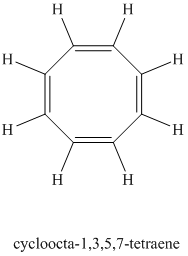
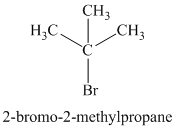
Explanation of Solution
Chemical formula:
A single peak at
Therefore, the structure of the compound is:
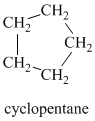
Chemical formula:
A single peak at
Therefore, it must be a symmetric cycloalkane:
Chemical formula:
A single peak at
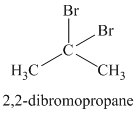
Chemical formula:
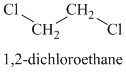
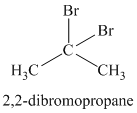
The chemical formula shows it is a saturated alkyl halide. A single peak means that all protons are equivalent. The presence of the bromine atoms accounts for the higher chemical shift of the protons. Therefore, the structure of the compound is:
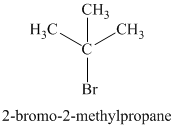
Chemical formula:
A single peak shows all protons to be equivalent. The formula is of a saturated alkyl halide. The presence of two chlorine atoms on the same carbon as the protons will increase the shift to
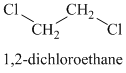
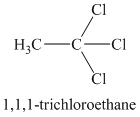
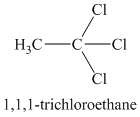
Chemical formula:
A single peak means all protons must be equivalent. The chemical formula shows it to be a saturated alkyl halide. The higher shift of
Chemical formula:
A single peak shows all protons to be equivalent. The chemical formula shows it to be a saturated alkyl halide. The high chemical shift of
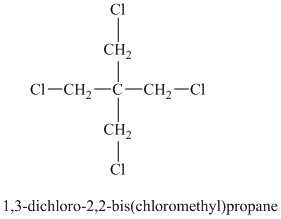
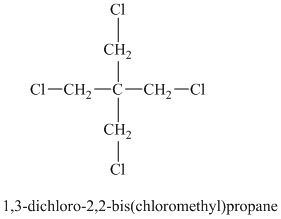
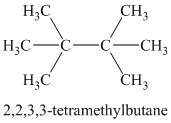
Chemical formula:
A single peak shows all protons are equivalent. The chemical formula shows an index of hydrogen deficiency of four. This, taken together with a chemical shift of
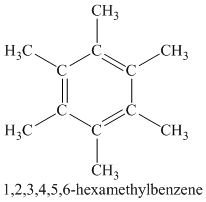
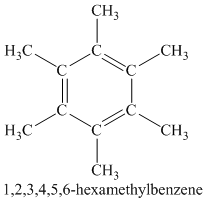
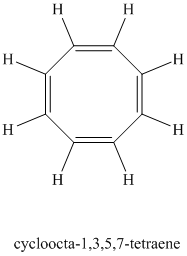
Chemical formula:
A single peak shows all protons are equivalent. The higher chemical shift of
Therefore, the structure of the compound is:
Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Please answer the questions and provide detailed explanations.arrow_forwardQuestion 16 0/1 pts Choose the correct option for the following cycloaddition reaction. C CF3 CF3 CF3 CF3 The reaction is suprafacial/surafacial and forbidden The reaction is antarafacial/antarafacial and forbidden The reaction is antarafacial/antarafacial and allowed The reaction is suprafacial/surafacial and allowedarrow_forward1. Give the structures of the products obtained when the following are heated. Include stereochemistry where relevant. A NO2 + NO2 B + C N=C CEN + { 2. Which compounds would you heat together in order to synthesize the following?arrow_forward
- Explain how myo-inositol is different from D-chiro-inositol. use scholarly sources and please hyperlink.arrow_forwardWhat is the molarisuty of a 0.396 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 /mol.arrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly. Br ......Im OHarrow_forward
- Can you please help me solve this problems. The top one is just drawing out the skeletal correct and then the bottom one is just very confusing to me and its quite small in the images. Can you enlarge it and explain it to me please. Thank You much (ME EX1) Prblm #33arrow_forwardI'm trying to memorize VESPR Shapes to solve problems like those. I need help making circles like the second image in blue or using an x- and y-axis plane to memorize these and solve those types of problems, especially the ones given in the top/first image (180, 120, 109.5). Can you help me with this? or is their any other efficient method do soarrow_forwardCan you please explain this problems to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 27arrow_forward
- Question 6 of 7 (1 point) | Question Attempt: 1 of 1 = 1 ✓2 ✓ 3 ✓ 4 ✓ 5 6 ✓ 7 This organic molecule is dissolved in a basic aqueous solution: Jen ✓ ? A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is, must now be a new molecule present with at least one C- OH bond. there 18 In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule Ar © + Click and drag to start drawing a structure. Add/Remove step Click and drawing Save For Later Submit Assignmentarrow_forwardCan you please explain this problem to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 22arrow_forwardCan you please explain this problems to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 30arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
