
EBK BASIC CHEMISTRY
6th Edition
ISBN: 9780134987088
Author: Timberlake
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.4, Problem 22PP
Use the following graph for problems 1.21 and 1.22: Temperature of Tea versus Time for Cooling
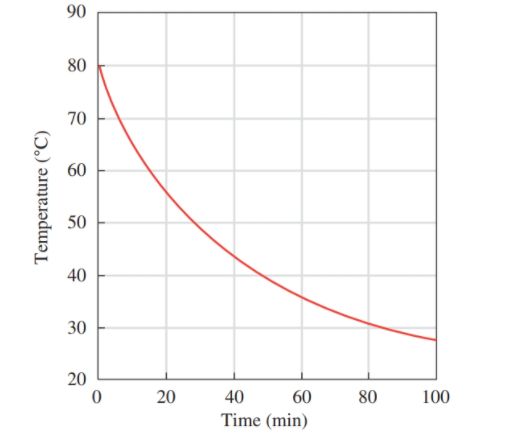
a. What is measured on the horizontal axis?
b. What is the range of values on the horizontal axis?
c. What is the temperature of the tea after 20 mm?
d. How many minutes were needed to reach a temperature of 45 °C
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Seee the attached ima
Please see the attached image.
Please see the attached image.
Chapter 1 Solutions
EBK BASIC CHEMISTRY
Ch. 1.1 - In every chapter. odd-numbered exercises in the...Ch. 1.1 - In every chapter. odd-numbered exercises in the...Ch. 1.1 - Prob. 3PPCh. 1.1 - Prob. 4PPCh. 1.1 - In every chapter. odd-numbered exercises in the...Ch. 1.1 - Prob. 6PPCh. 1.2 - Identify each activity, a to f, as an observation,...Ch. 1.2 - Identify each activity, a to f. as an observation,...Ch. 1.2 - Identify each of the following as an observation,...Ch. 1.2 - Identify each of the following as an observation,...
Ch. 1.3 - Prob. 11PPCh. 1.3 - What are four things that would make it difficult...Ch. 1.3 - Prob. 13PPCh. 1.3 - Prob. 14PPCh. 1.4 - What is the place value for the bold digit? a....Ch. 1.4 - What is the place value for the bold digit? a....Ch. 1.4 - Prob. 17PPCh. 1.4 - Prob. 18PPCh. 1.4 - Prob. 19PPCh. 1.4 - Prob. 20PPCh. 1.4 - Use the following graph for problems 1.21 and...Ch. 1.4 - Use the following graph for problems 1.21 and...Ch. 1.4 - a. A clinic had 25 patients on Friday morning....Ch. 1.4 - a. At a local hospital. 35 babies were born in...Ch. 1.4 - Prob. 25PPCh. 1.4 - Prob. 26PPCh. 1.5 - Prob. 27PPCh. 1.5 - Write each of the following in scientific...Ch. 1.5 - Prob. 29PPCh. 1.5 - Write each of the following as a standard number:...Ch. 1.5 - Prob. 31PPCh. 1.5 - Prob. 32PPCh. 1.5 - Prob. 33PPCh. 1.5 - Prob. 34PPCh. 1 - The chapter sections to review are shown in...Ch. 1 - Prob. 36UTCCh. 1 - Prob. 37UTCCh. 1 - Prob. 38UTCCh. 1 - Prob. 39UTCCh. 1 - Prob. 40UTCCh. 1 - Prob. 41UTCCh. 1 - Prob. 42UTCCh. 1 - Select the correct phrase(s) to complete the...Ch. 1 - Select the correct phrase(s) to complete the...Ch. 1 - Prob. 45APPCh. 1 - Prob. 46APPCh. 1 - Prob. 47APPCh. 1 - Prob. 48APPCh. 1 - Evaluate each of the following: (1.4)...Ch. 1 - Prob. 50APPCh. 1 - A bag of gumdrops contains 16 orange gumdrops, 8...Ch. 1 - Prob. 52APPCh. 1 - Write each of the following in scientific...Ch. 1 - Write each of the following in scientific...Ch. 1 - Prob. 55APPCh. 1 - Write each of the following as a standard number:...Ch. 1 - Prob. 57APPCh. 1 - Prob. 58APPCh. 1 - Prob. 59CPCh. 1 - Prob. 60CPCh. 1 - Prob. 61CPCh. 1 - The following problems are related to the topics...Ch. 1 - The following problems are related to the topics...Ch. 1 - Prob. 64CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY